Nuclear Stability | Chemistry for ACT PDF Download
Introduction
While the majority of known elements possess at least one isotope with a stable atomic nucleus that remains intact indefinitely, there are isotopes for all elements that are unstable and undergo decay at detectable rates, emitting radiation in the process. Certain elements do not have any stable isotopes and eventually decay into different elements. Unlike the chemical reactions discussed in previous chapters, which primarily involve alterations in the arrangement of valence electrons within atoms, nuclear decay involves changes occurring within the atomic nucleus itself.
The Atomic Nucleus
Each element can be represented by the notation 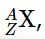 The mass number (A) of an atom is defined as the sum of the number of protons and neutrons present in its nucleus, while the atomic number (Z) corresponds to the number of protons. The protons and neutrons within the atomic nucleus are collectively referred to as nucleons, and an atom with a specific count of protons and neutrons is known as a nuclide. Nuclides that have the same number of protons but varying numbers of neutrons are called isotopes. Isotopes can also be denoted using an alternative notation that includes the element's name followed by the mass number, such as carbon-12. For instance, the stable isotopes of oxygen can be represented using any of the following formats:
The mass number (A) of an atom is defined as the sum of the number of protons and neutrons present in its nucleus, while the atomic number (Z) corresponds to the number of protons. The protons and neutrons within the atomic nucleus are collectively referred to as nucleons, and an atom with a specific count of protons and neutrons is known as a nuclide. Nuclides that have the same number of protons but varying numbers of neutrons are called isotopes. Isotopes can also be denoted using an alternative notation that includes the element's name followed by the mass number, such as carbon-12. For instance, the stable isotopes of oxygen can be represented using any of the following formats: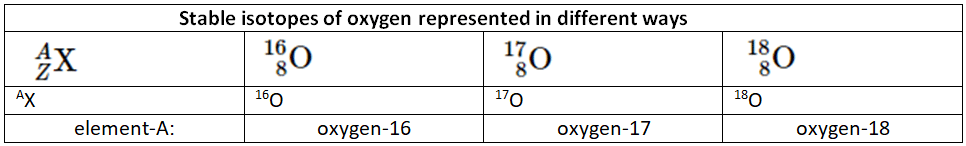
By subtracting the atomic number (Z) from the mass number (A), we can determine the number of neutrons in an atom. Applying this formula, we find that the first isotope of oxygen contains 8 neutrons, the second isotope contains 9 neutrons, and the third isotope contains 10 neutrons. Naturally occurring elements on Earth have isotopes present in relatively fixed proportions, with each proportion representing the natural abundance of a specific isotope. For instance, in a typical sample of oxygen found on Earth, 99.76% of oxygen atoms are oxygen-16, 0.20% are oxygen-18, and 0.04% are oxygen-17.
Nuclei that are unstable and spontaneously decay are classified as radioactive, emitting subatomic particles and electromagnetic radiation. The collective term for these emissions is radioactivity, and it can be measured. Isotopes that emit radiation are referred to as radioisotopes.
Nuclear Stability
The nucleus of an atom is situated within a minuscule portion of the atom's overall volume and holds the characteristic number of protons and neutrons specific to a given isotope. Ordinarily, electrostatic repulsion would cause the positively charged protons to repel each other, but the nucleus remains intact due to the presence of the strong nuclear force. This force is incredibly potent but has a very short range, acting as an attractive force between nucleons to counterbalance the repulsion (as shown in Figure 1). With the exception of the hydrogen-1 nucleus (1H), all stable nuclei contain at least one neutron to counteract the electrostatic repulsion between protons. As the number of protons in the nucleus increases, the requirement for an increased number of neutrons to maintain stability rises even more rapidly. An excess of protons (or a deficiency of neutrons) within the nucleus leads to an imbalance of forces, resulting in nuclear instability.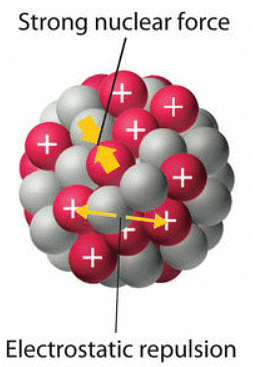
Figure 1: Competing Interactions within the Atomic Nucleus: Electrostatic repulsions between positively charged protons would normally cause the nuclei of atoms (except H) to fly apart. In stable atomic nuclei, these repulsions are overcome by the strong nuclear force, a short-range but powerful attractive interaction between nucleons. If the attractive interactions due to the strong nuclear force are weaker than the electrostatic repulsions between protons, the nucleus is unstable, and it will eventually decay.
The correlation between the number of protons and the number of neutrons in stable nuclei, which are defined as having a half-life longer than 10 times the age of Earth, is depicted visually in Figure 2. Within this depiction, the stable isotopes form a distinct region known as the "peninsula of stability" amidst an encompassing "sea of instability." Only two stable isotopes, 1H and 3He, possess a neutron-to-proton ratio less than 1. Additionally, several stable isotopes of lighter elements exhibit a neutron-to-proton ratio that is equal to 1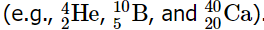 . All other stable nuclei have a higher neutron-to-proton ratio, which increases steadily to about 1.5 for the heaviest nuclei. Regardless of the number of neutrons, however, all elements with Z > 83 are unstable and radioactive.
. All other stable nuclei have a higher neutron-to-proton ratio, which increases steadily to about 1.5 for the heaviest nuclei. Regardless of the number of neutrons, however, all elements with Z > 83 are unstable and radioactive.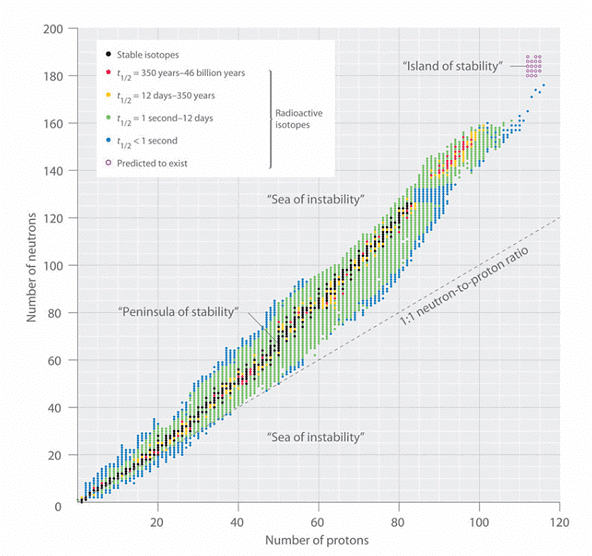 Figure 2: The Relationship between Nuclear Stability and the Neutron-to-Proton Ratio: In this plot of the number of neutrons versus the number of protons, each black point corresponds to a stable nucleus. In this classification, a stable nucleus is arbitrarily defined as one with a half-life longer than 46 billion years (10 times the age of Earth). As the number of protons (the atomic number) increases, the number of neutrons required for a stable nucleus increases even more rapidly. Isotopes shown in red, yellow, green, and blue are progressively less stable and more radioactive; the farther an isotope is from the diagonal band of stable isotopes, the shorter its half-life. The purple dots indicate superheavy nuclei that are predicted to be relatively stable, meaning that they are expected to be radioactive but to have relatively long half-lives. In most cases, these elements have not yet been observed or synthesized.
Figure 2: The Relationship between Nuclear Stability and the Neutron-to-Proton Ratio: In this plot of the number of neutrons versus the number of protons, each black point corresponds to a stable nucleus. In this classification, a stable nucleus is arbitrarily defined as one with a half-life longer than 46 billion years (10 times the age of Earth). As the number of protons (the atomic number) increases, the number of neutrons required for a stable nucleus increases even more rapidly. Isotopes shown in red, yellow, green, and blue are progressively less stable and more radioactive; the farther an isotope is from the diagonal band of stable isotopes, the shorter its half-life. The purple dots indicate superheavy nuclei that are predicted to be relatively stable, meaning that they are expected to be radioactive but to have relatively long half-lives. In most cases, these elements have not yet been observed or synthesized.
Graph of number or neutrons against the number or protons. The graph is divided into sections of "sea of instability", "peninsula of stability", "sea of instability" and "island of stability".
As shown in Figure 3, more than half of the stable nuclei (166 out of 279) have even numbers of both neutrons and protons; only 6 of the 279 stable nuclei do not have odd numbers of both. Moreover, certain numbers of neutrons or protons result in especially stable nuclei; these are the so-called magic numbers 2, 8, 20, 50, 82, and 126. For example, tin (Z = 50) has 10 stable isotopes, but the elements on either side of tin in the periodic table, indium (Z = 49) and antimony (Z = 51), have only 2 stable isotopes each. Nuclei with magic numbers of both protons and neutrons are said to be “doubly magic” and are even more stable. Examples of elements with doubly magic nuclei are 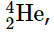 with 2 protons and 2 neutrons, and
with 2 protons and 2 neutrons, and 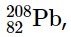 with 82 protons and 126 neutrons, which is the heaviest known stable isotope of any element.
with 82 protons and 126 neutrons, which is the heaviest known stable isotope of any element.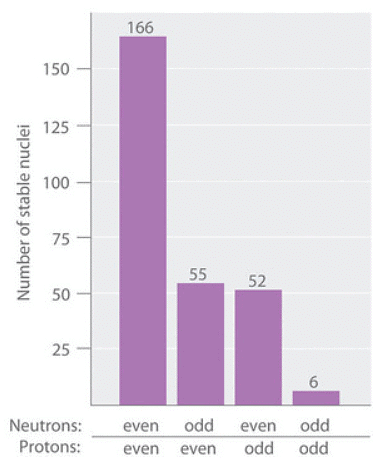
Figure 3: The Relationship between the Number of Protons and the Number of Neutrons and Nuclear Stability.
Most stable nuclei contain even numbers of both neutrons and protons.
The stability pattern observed in relation to the magic numbers of nucleons bears resemblance to the stability seen in the closed-shell electron configurations of noble gases found in Group 18. This resemblance has prompted the hypothesis that the atomic nucleus possesses nucleon shells that are somewhat analogous to the electron shells occupied in an atom. As depicted in Figure 2, the region referred to as the "peninsula" encompasses stable isotopes, while surrounding it is a "reef" consisting of radioactive isotopes. These radioactive isotopes have varying degrees of stability, with lifetimes before eventual decay and transformation into other nuclei.
Example 1: Classify each nuclide as stable or radioactive.
(a) 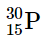
(b) 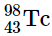
(c) tin-118
(d) 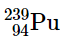
Given: mass number and atomic number
Asked for: predicted nuclear stability
Strategy: Use the number of protons, the neutron-to-proton ratio, and the presence of even or odd numbers of neutrons and protons to predict the stability or radioactivity of each nuclide.
(a) This isotope of phosphorus has 15 neutrons and 15 protons, giving a neutron-to-proton ratio of 1.0. Although the atomic number, 15, is much less than the value of 83 above which all nuclides are unstable, the neutron-to-proton ratio is less than that expected for stability for an element with this mass. As shown in Figure 2, its neutron-to-proton ratio should be greater than 1. Moreover, this isotope has an odd number of both neutrons and protons, which also tends to make a nuclide unstable. Consequently,
is predicted to be radioactive, and it is.
(b) This isotope of technetium has 55 neutrons and 43 protons, giving a neutron-to-proton ratio of 1.28, which placesnear the edge of the band of stability. The atomic number, 55, is much less than the value of 83 above which all isotopes are unstable. These facts suggest that
might be stable. However,
has an odd number of both neutrons and protons, a combination that seldom gives a stable nucleus. Consequently,
is predicted to be radioactive, and it is.
(c) Tin-118 has 68 neutrons and 50 protons, for a neutron-to-proton ratio of 1.36. As in part b, this value and the atomic number both suggest stability. In addition, the isotope has an even number of both neutrons and protons, which tends to increase nuclear stability. Most important, the nucleus has 50 protons, and 50 is one of the magic numbers associated with especially stable nuclei. Thusshould be particularly stable.
(d) This nuclide has an atomic number of 94. Because all nuclei with Z > 83 are unstable,must be radioactive.
Example 2: Classify each nuclide as stable or radioactive.
(a) 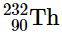
(b) 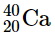
(c) 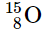
(d) 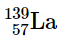
(a) radioactive
(b) stable
(c) radioactive
(d) stable
Superheavy Elements
In addition to the recognized "peninsula of stability," there is a smaller region known as the "island of stability" that is predicted to exist in the upper right corner. This island corresponds to superheavy elements with atomic numbers close to the magic number 126. It was proposed that an element with 114 protons and 184 neutrons could potentially be stable since the next magic number for neutrons is expected to be 184. These claims faced skepticism for a long time. However, starting from 1999, a few atoms of isotopes with Z = 114 and Z = 116 have been successfully synthesized and found to exhibit surprising stability. One specific isotope of element 114 has a relatively long lifespan of 2.7 seconds before undergoing decay, which is considered an impressive duration in the field of nuclear chemistry. Additionally, recent evidence suggests the existence of a nucleus with A = 292, discovered within 232Th. This isotope exhibits remarkable stability with an estimated half-life exceeding 108 years. The measured mass of this nucleus aligns with predictions for an isotope with Z = 122. These findings indicate that a number of relatively long-lived nuclei might be within reach among the superheavy elements.
Summary
Particles found within the atomic nucleus, namely protons and neutrons, are referred to as nucleons. A nuclide represents an atom characterized by a specific count of protons and neutrons. When a nucleus is unstable and undergoes spontaneous decay, it is considered radioactive, and the emissions it releases are collectively known as radioactivity. Isotopes that emit radiation are called radioisotopes. The strong nuclear force attracts each nucleon to other nucleons within the nucleus. Stable nuclei typically possess an even number of both protons and neutrons, with a neutron-to-proton ratio of at least 1. Nuclei containing magic numbers of protons and neutrons often exhibit heightened stability. It is even possible that superheavy elements with atomic numbers close to 126 may possess sufficient stability to exist in the natural world.
|
110 videos|141 docs|120 tests
|




















