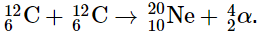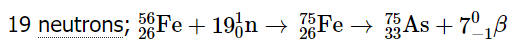Nuclear Transmutations | Chemistry for ACT PDF Download
Introduction
The relative abundances of elements found in the universe exhibit substantial variations spanning more than 12 orders of magnitude. These discrepancies in abundance cannot be solely attributed to differences in nuclear stability. Despite the 56Fe nucleus being the most stable known nucleus, the most prevalent element in the universe is not iron but rather hydrogen (1H), constituting approximately 90% of all atoms. In fact, hydrogen serves as the fundamental building block from which all other elements are formed. In this section, we delve into an explanation for why hydrogen (1H) and helium-2 (2He) collectively account for at least 99% of all atoms within the known universe. Furthermore, we elucidate the nuclear reactions occurring within stars, which facilitate the conversion of one nucleus to another and give rise to all naturally occurring elements.
Relative Abundances of the Elements on Earth and in the Known Universe
Figure 1 presents the relative abundance of elements in the known universe and on Earth, with silicon serving as a reference point. These estimates are derived from various sources, including the emission spectra of elements in stars, the absorption spectra of matter in interstellar dust clouds, and the composition of Earth as determined by geologists. The data in Figure 1 highlight two significant observations. Firstly, apart from hydrogen, the most abundant elements tend to have even atomic numbers. This observation aligns with the well-established patterns of nuclear stability and suggests that heavier elements are formed through the fusion of helium nuclei (Z = 2). Secondly, the relative abundances of elements differ significantly between the known universe and Earth, as indicated by the data in Table 1, which provides information on the relative abundances of certain common elements.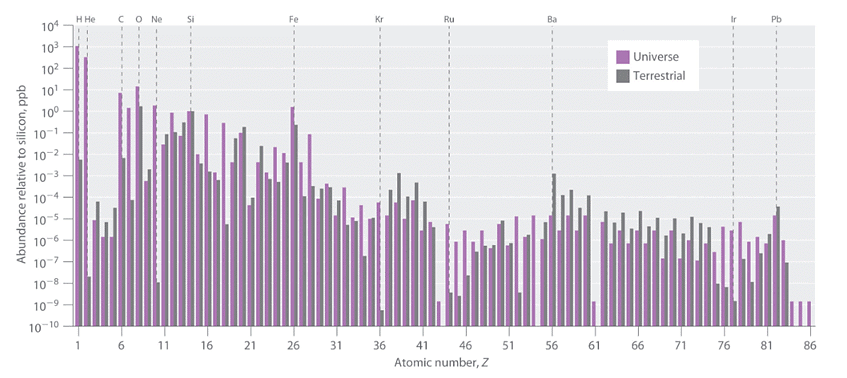 Figure 1: The Relative Abundances of the Elements in the Universe and on Earth: In this logarithmic plot, the relative abundances of the elements relative to that of silicon (arbitrarily set equal to 1) in the universe (green bars) and on Earth (purple bars) are shown as a function of atomic number. Elements with even atomic numbers are generally more abundant in the universe than elements with odd atomic numbers. Also, the relative abundances of many elements in the universe are very different from their relative abundances on Earth.
Figure 1: The Relative Abundances of the Elements in the Universe and on Earth: In this logarithmic plot, the relative abundances of the elements relative to that of silicon (arbitrarily set equal to 1) in the universe (green bars) and on Earth (purple bars) are shown as a function of atomic number. Elements with even atomic numbers are generally more abundant in the universe than elements with odd atomic numbers. Also, the relative abundances of many elements in the universe are very different from their relative abundances on Earth.
Bar graph of abundance realtive to silicon in pbb against atomic number. The purple bars are universe elements while the gray bars are terrestrial elements.
Certain disparities in elemental abundances can be easily explained. On Earth, nonmetals like hydrogen (H), helium (He), carbon (C), nitrogen (N), oxygen (O), neon (Ne), and krypton (Kr) are considerably less abundant relative to silicon compared to their prevalence in the rest of the universe. These elements comprise either noble gases (He, Ne, and Kr) or elements that form volatile hydrides, such as ammonia (NH3), methane (CH4), and water (H2O). Due to Earth's relatively weak gravity, these lightweight substances have gradually diffused into outer space since the planet's formation, as they are unable to be retained in the atmosphere. Argon is an exception to this trend as it is relatively abundant on Earth compared to other noble gases.
This is primarily because argon is continuously generated within rocks through the radioactive decay of isotopes like potassium-40 (40K). Conversely, numerous metals such as aluminum (Al), sodium (Na), iron (Fe), calcium (Ca), magnesium (Mg), potassium (K), and titanium (Ti) are relatively abundant on Earth as they form nonvolatile compounds, such as oxides, which are unable to escape into space. On the other hand, certain metals like ruthenium (Ru) and iridium (Ir) are significantly less abundant on Earth compared to their prevalence in the universe. This section elucidates some of the underlying reasons behind these substantial variations in the abundance of metallic elements.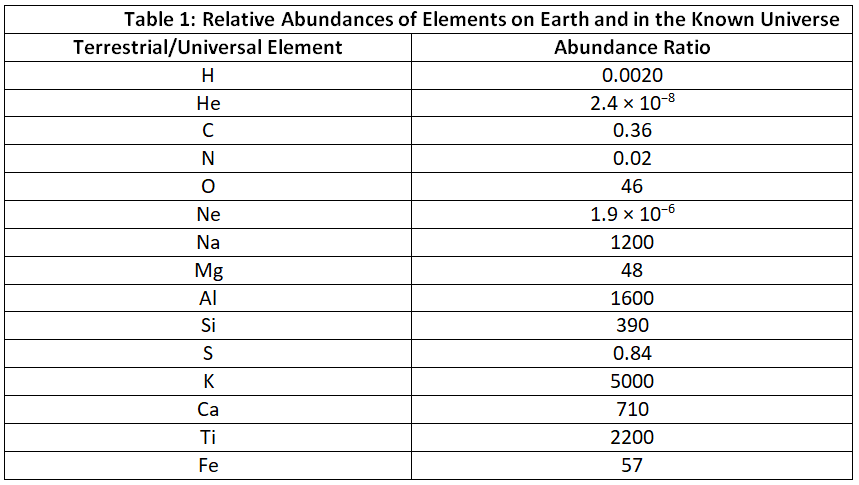 The elements found on Earth, as well as other planets, were initially created through the fusion of hydrogen and helium nuclei within stars that have since undergone supernovae and ceased to exist. Among the most prevalent elements in the universe, including carbon (C), oxygen (O), neon (Ne), magnesium (Mg), silicon (Si), and iron (Fe), their nuclei consist of multiple helium-4 nuclei, indicating that helium-4 serves as the fundamental constituent for the formation of heavier atomic nuclei.
The elements found on Earth, as well as other planets, were initially created through the fusion of hydrogen and helium nuclei within stars that have since undergone supernovae and ceased to exist. Among the most prevalent elements in the universe, including carbon (C), oxygen (O), neon (Ne), magnesium (Mg), silicon (Si), and iron (Fe), their nuclei consist of multiple helium-4 nuclei, indicating that helium-4 serves as the fundamental constituent for the formation of heavier atomic nuclei.
Synthesis of the Elements in Stars
The elements found on Earth, as well as other planets, were initially created through the fusion of hydrogen and helium nuclei within stars that have since undergone supernovae and ceased to exist. Among the most prevalent elements in the universe, including carbon (C), oxygen (O), neon (Ne), magnesium (Mg), silicon (Si), and iron (Fe), their nuclei consist of multiple helium-4 nuclei, indicating that helium-4 serves as the fundamental constituent for the formation of heavier atomic nuclei.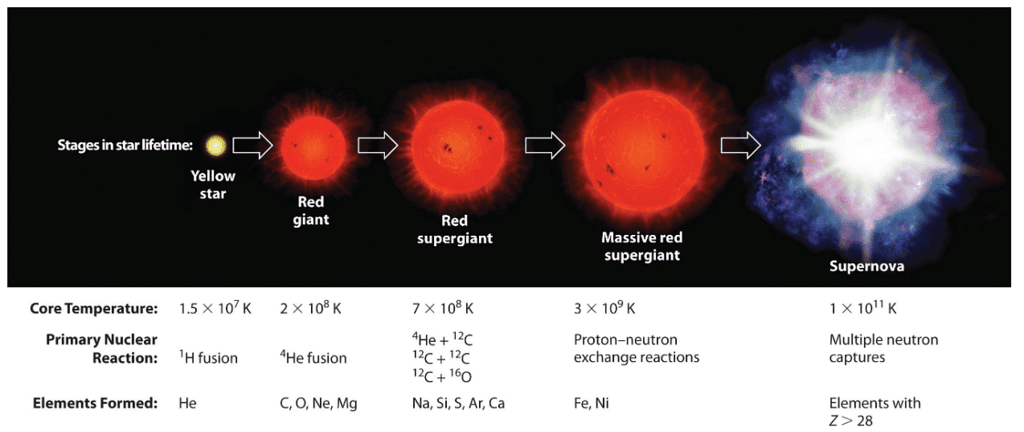
Figure 2: illustrates the sequence of nuclear reactions that occur throughout the life cycle of a massive star. Each stage of the star's life involves the utilization of distinct fuels for nuclear fusion, leading to the production of different elements. In the early stages, when the star is young, the primary fusion reaction involves the conversion of hydrogen into helium. As the star progresses in age, helium gradually accumulates and undergoes fusion, giving rise to the creation of heavier elements like carbon and oxygen. During the later stages of the star's development, significant amounts of iron and nickel are synthesized through the fusion of previously formed heavier elements. The synthesis of the heaviest elements occurs exclusively during the star's final stages, characterized by events like novae or supernovae, which mark the star's ultimate demise.
The stages in a star lifetime are yellow star, red giant, red supergiant, massive red supergiant and finally supernova.
In the first stage of its life, the star is powered by a series of nuclear fusion reactions that convert hydrogen to helium: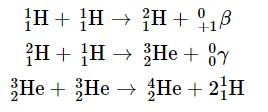 The overall reaction is the conversion of four hydrogen nuclei to a helium-4 nucleus, which is accompanied by the release of two positrons, two γ rays, and a great deal of energy:
The overall reaction is the conversion of four hydrogen nuclei to a helium-4 nucleus, which is accompanied by the release of two positrons, two γ rays, and a great deal of energy: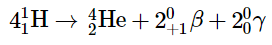
These reactions are responsible for most of the enormous amount of energy that is released as sunlight and solar heat. It takes several billion years, depending on the size of the star, to convert about 10% of the hydrogen to helium.
Once large amounts of helium-4 have been formed, they become concentrated in the core of the star, which slowly becomes denser and hotter. At a temperature of about 2 × 108 K, the helium-4 nuclei begin to fuse, producing beryllium-8: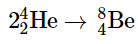 Although beryllium-8 has both an even mass number and an even atomic number, its also has a low neutron-to-proton ratio (and other factors beyond the scope of this text) that makes it unstable; it decomposes in only about 10−16 s. Nonetheless, this is long enough for it to react with a third helium-4 nucleus to form carbon-12, which is very stable. Sequential reactions of carbon-12 with helium-4 produce the elements with even numbers of protons and neutrons up to magnesium-24:
Although beryllium-8 has both an even mass number and an even atomic number, its also has a low neutron-to-proton ratio (and other factors beyond the scope of this text) that makes it unstable; it decomposes in only about 10−16 s. Nonetheless, this is long enough for it to react with a third helium-4 nucleus to form carbon-12, which is very stable. Sequential reactions of carbon-12 with helium-4 produce the elements with even numbers of protons and neutrons up to magnesium-24: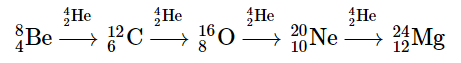 So much energy is released by these reactions that it causes the surrounding mass of hydrogen to expand, producing a red giant that is about 100 times larger than the original yellow star.
So much energy is released by these reactions that it causes the surrounding mass of hydrogen to expand, producing a red giant that is about 100 times larger than the original yellow star.
As the star expands, heavier nuclei accumulate in its core, which contracts further to a density of about 50,000 g/cm3, so the core becomes even hotter. At a temperature of about 7 × 108 K, carbon and oxygen nuclei undergo nuclear fusion reactions to produce sodium and silicon nuclei: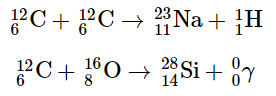
At these temperatures, carbon-12 reacts with helium-4 to initiate a series of reactions that produce more oxygen-16, neon-20, magnesium-24, and silicon-28, as well as heavier nuclides such as sulfur-32, argon-36, and calcium-40: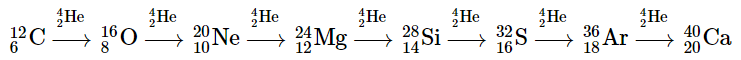
The energy released by these reactions causes a further expansion of the star to form a red supergiant, and the core temperature increases steadily. At a temperature of about 3 × 109 K, the nuclei that have been formed exchange protons and neutrons freely. This equilibration process forms heavier elements up to iron-56 and nickel-58, which have the most stable nuclei known.
The Formation of Heavier Elements in Supernovas
All elements beyond atomic number 28 (nickel) are not naturally formed through the processes described earlier. Instead, they are synthesized during the infrequent yet remarkable cataclysmic explosions known as supernovae (depicted in Figure 2). When the core of an exceptionally massive star exhausts its fuel, gravitational forces cause it to collapse rapidly in approximately one second. During this compression, iron and nickel nuclei in the core disintegrate into protons and neutrons, with many protons capturing electrons to become neutrons.
The resulting compact object, known as a neutron star, is incredibly dense, eliminating the presence of atoms. Concurrently, the tremendous energy released from the core's collapse triggers an explosion, unleashing a supernova—an event considered to be one of the most violent occurrences in the universe. The explosion propels the majority of the star's matter into space, generating an enormous and swiftly expanding dust cloud or nebula (as depicted in Figure 3). Within the exceptionally brief duration of this phenomenon, the concentration of neutrons becomes extremely high, enabling multiple neutron-capture events to take place. Consequently, the heaviest elements and numerous less stable isotopes are produced. In such conditions, an iron-56 nucleus can absorb up to 64 neutrons temporarily, resulting in an extraordinarily unstable isotope of iron. This isotope then undergoes rapid β-decay processes, ultimately yielding tin-120.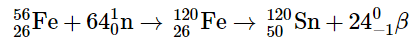
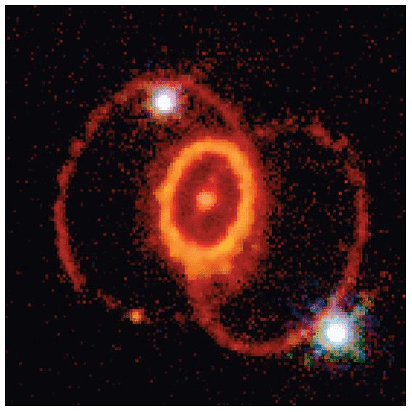 Figure 3: A Supernova: A view of the remains of Supernova 1987A, located in the Large Magellanic Cloud, showing the circular halo of expanding debris produced by the explosion. Multiple neutron-capture events occur during a supernova explosion, forming both the heaviest elements and many of the less stable nuclides.
Figure 3: A Supernova: A view of the remains of Supernova 1987A, located in the Large Magellanic Cloud, showing the circular halo of expanding debris produced by the explosion. Multiple neutron-capture events occur during a supernova explosion, forming both the heaviest elements and many of the less stable nuclides.
Supernovae, although infrequent events occurring every few hundred years in a galaxy like the Milky Way, are the sole occurrences that allow for the creation of elements beyond nickel. The immense power unleashed by these rare explosions disperses these heavy elements throughout the surrounding galaxy, where they become embedded in the interstellar dust and subsequently incorporated into newly forming stars. Our sun, based on its elemental composition, is believed to be a star that has formed from the remnants of previous supernova explosions, making it a second- or third-generation star. It contains a significant amount of cosmic debris originating from the distant past supernova events.
Example 1: The reaction of two carbon-12 nuclei in a carbon-burning star can produce elements other than sodium. Write a balanced nuclear equation for the formation of
(a) magnesium-24.
(b) neon-20 from two carbon-12 nuclei.
Given: reactant and product nuclides
Asked for: balanced nuclear equation
Strategy: Use conservation of mass and charge to determine the type of nuclear reaction that will convert the reactant to the indicated product. Write the balanced nuclear equation for the reaction.
(a) A magnesium-24 nucleus (Z = 12, A = 24) has the same nucleons as two carbon-12 nuclei (Z = 6, A = 12). The reaction is therefore a fusion of two carbon-12 nuclei, and no other particles are produced:
(b) The neon-20 product has Z = 10 and A = 20. The conservation of mass requires that the other product have A = (2 × 12) − 20 = 4; because of conservation of charge, it must have Z = (2 × 6) − 10 = 2. These are the characteristics of an α particle. The reaction is therefore
Example 2: How many neutrons must an iron-56 nucleus absorb during a supernova explosion to produce an arsenic-75 nucleus? Write a balanced nuclear equation for the reaction.
Summary
Hydrogen and helium are the most abundant elements found throughout the universe. The formation of heavier elements takes place within stars, where multiple neutron-capture events play a crucial role. Hydrogen is the most prevalent element in the universe, and the fusion of hydrogen nuclei into helium nuclei serves as the primary source of energy for young stars like our sun. Elements heavier than helium are synthesized within the cores of stars through successive fusion reactions. This process involves the fusion of helium nuclei at increasingly higher temperatures, leading to the creation of elements with even numbers of protons and neutrons, including elements like magnesium and calcium. As temperatures continue to rise, exchange processes give rise to the formation of elements up to iron-56 and nickel-58. However, the production of heavier elements necessitates a different mechanism, one that involves multiple neutron-capture events. Such events occur exclusively during the explosive phenomenon of a supernova.
|
110 videos|130 docs|117 tests
|