Nuclear Fission | Chemistry for ACT PDF Download
Introduction
Elements with lower binding energies per nucleon and larger mass numbers can undergo fission, a process in which a large nucleus breaks apart into smaller fragments. This decomposition results in the formation of more stable elements with intermediate mass numbers and higher binding energies per nucleon, closer to the peak of the binding energy graph around 56. Occasionally, neutrons are also released during fission. While fission does not typically occur naturally, it can be induced by bombarding the nucleus with neutrons. The first documented case of nuclear fission took place in 1939 when Lise Meitner, Otto Hahn, and Fritz Strassman, German scientists, bombarded uranium-235 atoms with slow-moving neutrons, causing the U-238 nuclei to split into smaller fragments composed of several neutrons and elements near the middle of the periodic table. Since then, fission has been observed in various isotopes, particularly in actinide isotopes that possess an odd number of neutrons. Figure 1 illustrates a typical nuclear fission reaction. Figure 1: When a slow neutron hits a fissionable U-235 nucleus, it is absorbed and forms an unstable U-236 nucleus. The U-236 nucleus then rapidly breaks apart into two smaller nuclei (in this case, Ba-141 and Kr-92) along with several neutrons (usually two or three), and releases a very large amount of energy.
Figure 1: When a slow neutron hits a fissionable U-235 nucleus, it is absorbed and forms an unstable U-236 nucleus. The U-236 nucleus then rapidly breaks apart into two smaller nuclei (in this case, Ba-141 and Kr-92) along with several neutrons (usually two or three), and releases a very large amount of energy.
Barium, krypton, lanthanum, and cerium were among the products generated in the fission reaction conducted by Meitner, Hahn, and Strassman. These elements possess nuclei that are more stable compared to uranium-235. Subsequently, numerous isotopes have been identified as products of fissionable substances, resulting in a wide range of reactions. Figure 2 presents a selection of reactions and a graph illustrating the distribution of fission products and their corresponding yields specifically for U-235. Additionally, similar fission reactions have been observed not only with other uranium isotopes but also with various isotopes, including plutonium. Figure 2: (a) Nuclear fission of U-235 produces a range of fission products.
Figure 2: (a) Nuclear fission of U-235 produces a range of fission products.
(b) The larger fission products of U-235 are typically one isotope with a mass number around 85–105, and another isotope with a mass number that is about 50% larger, that is, about 130–150.
The fission of heavy elements results in an immense release of energy. To illustrate, when one mole of U-235 undergoes fission, the resulting products weigh approximately 0.2 grams less than the initial reactants. This apparent loss in mass is actually converted into an extremely large quantity of energy, estimated to be around 1.8 × 1010 kJ per mole of U-235. Nuclear fission reactions generate an extraordinary amount of energy in comparison to chemical reactions. As an example, the fission of 1 kilogram of uranium-235 produces approximately 2.5 million times more energy than the combustion of 1 kilogram of coal.
As previously explained, during fission, U-235 yields two intermediate-sized nuclei and two or three neutrons. These neutrons have the potential to induce the fission of additional U-235 atoms, releasing more neutrons that can in turn trigger the fission of even more nuclei, and so forth. When this process takes place, a nuclear chain reaction ensues, as depicted in Figure 3. Conversely, if a significant number of neutrons escape the material without interacting with a nucleus, a chain reaction will not be sustained.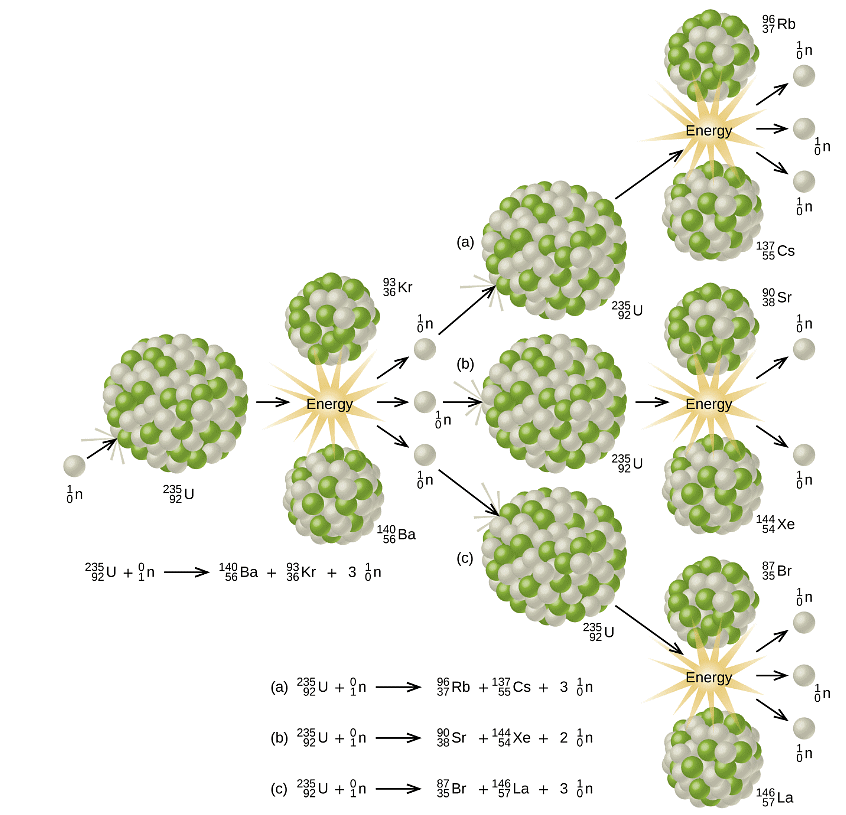 Figure 3: The fission of a large nucleus, such as U-235, produces two or three neutrons, each of which is capable of causing fission of another nucleus by the reactions shown. If this process continues, a nuclear chain reaction occurs.
Figure 3: The fission of a large nucleus, such as U-235, produces two or three neutrons, each of which is capable of causing fission of another nucleus by the reactions shown. If this process continues, a nuclear chain reaction occurs.
A substance capable of maintaining a continuous nuclear fission chain reaction is referred to as fissile or fissionable. Specifically, fissile material can undergo fission with neutrons of any energy, whereas fissionable material requires high-energy neutrons. The self-sustainability of nuclear fission occurs when the number of neutrons generated by fission is equal to or greater than the number of neutrons absorbed by splitting nuclei, in addition to the number of neutrons that escape into the surroundings.
The quantity of fissionable material required to sustain a self-sustaining chain reaction is known as the critical mass. Conversely, an amount of fissionable material that cannot sustain a chain reaction is classified as a subcritical mass. When the rate of fission increases within a certain quantity of material, it is referred to as a supercritical mass. The critical mass is influenced by various factors such as the material's purity, temperature, the shape of the sample, and the control of neutron reactions, as depicted in Figure 4. Figure 4: (a) In a subcritical mass, the fissile material is too small and allows too many neutrons to escape the material, so a chain reaction does not occur.
Figure 4: (a) In a subcritical mass, the fissile material is too small and allows too many neutrons to escape the material, so a chain reaction does not occur.
(b) In a critical mass, a large enough number of neutrons in the fissile material induce fission to create a chain reaction.
An atomic bomb, illustrated in Figure 4, consists of a few pounds of fissionable material, such as  , along with a neutron source and an explosive device that rapidly compresses the material into a compact volume. In the initial state, when the fissionable material is divided into small pieces, a significant proportion of neutrons escape through the relatively large surface area, preventing a chain reaction from occurring. However, when these small pieces are swiftly brought together to form a larger mass exceeding the critical mass, the number of escaping neutrons decreases proportionately. As a result, a chain reaction is triggered, leading to an explosive release of energy.
, along with a neutron source and an explosive device that rapidly compresses the material into a compact volume. In the initial state, when the fissionable material is divided into small pieces, a significant proportion of neutrons escape through the relatively large surface area, preventing a chain reaction from occurring. However, when these small pieces are swiftly brought together to form a larger mass exceeding the critical mass, the number of escaping neutrons decreases proportionately. As a result, a chain reaction is triggered, leading to an explosive release of energy. Figure 5: (a) The nuclear fission bomb that destroyed Hiroshima on August 6, 1945, consisted of two subcritical masses of U-235, where conventional explosives were used to fire one of the subcritical masses into the other, creating the critical mass for the nuclear explosion.
Figure 5: (a) The nuclear fission bomb that destroyed Hiroshima on August 6, 1945, consisted of two subcritical masses of U-235, where conventional explosives were used to fire one of the subcritical masses into the other, creating the critical mass for the nuclear explosion.
(b) The plutonium bomb that destroyed Nagasaki on August 12, 1945, consisted of a hollow sphere of plutonium that was rapidly compressed by conventional explosives. This led to a concentration of plutonium in the center that was greater than the critical mass necessary for the nuclear explosion.
Fission Reactors
Nuclear reactors, as depicted in Figure 6, provide a controlled and sustained chain reaction of fissionable materials without causing an explosion. For a nuclear reactor to generate power through the fission of uranium or plutonium by neutron bombardment, it requires five essential components: nuclear fuel containing fissionable material, a nuclear moderator, reactor coolant, control rods, and a shield and containment system. These components will be discussed in more detail later in this section. The reactor operates by preventing the formation of a critical mass of fissionable material, while simultaneously regulating the flux and absorption of neutrons to enable the shutdown of fission reactions. In an electricity-producing nuclear reactor, the energy released from fission reactions is captured as thermal energy, which is then utilized to heat water and produce steam. The steam drives a turbine that powers a generator, thus generating electricity. Figure 6: (a) The Diablo Canyon Nuclear Power Plant near San Luis Obispo is the only nuclear power plant currently in operation in California. The domes are the containment structures for the nuclear reactors, and the brown building houses the turbine where electricity is generated. Ocean water is used for cooling.
Figure 6: (a) The Diablo Canyon Nuclear Power Plant near San Luis Obispo is the only nuclear power plant currently in operation in California. The domes are the containment structures for the nuclear reactors, and the brown building houses the turbine where electricity is generated. Ocean water is used for cooling.
(b) The Diablo Canyon uses a pressurized water reactor, one of a few different fission reactor designs in use around the world, to produce electricity. Energy from the nuclear fission reactions in the core heats water in a closed, pressurized system. Heat from this system produces steam that drives a turbine, which in turn produces electricity.
Nuclear Fuels
Nuclear fuel is composed of a fissionable isotope, such as uranium-235, which needs to be present in sufficient quantity to sustain a self-sustaining chain reaction. In the United States, uranium ores typically contain about 0.05-0.3% of uranium oxide U3O8. The majority of uranium in the ore, approximately 99.3%, is nonfissionable U-238, with only 0.7% being the fissionable U-235 isotope. However, nuclear reactors require a higher concentration of U-235 than naturally occurring levels, and therefore the uranium fuel needs to be enriched. Typically, the fuel is enriched to contain around 5% of uranium mass as U-235. This level of enrichment is still far below the supercritical mass required for a nuclear explosion, ensuring the reactor's safety. There are several methods for enriching uranium, including gaseous diffusion (currently used in the US), gas centrifuge, and laser separation.
The gaseous diffusion enrichment plant prepares U-235 fuel by passing UF6 gas at low pressure through barriers with tiny holes. These holes are just big enough for the UF6 molecules to pass through. The lighter 235UF6 molecules diffuse slightly faster through the barriers compared to the heavier 238UF6 molecules. This process is repeated across numerous barriers, gradually increasing the concentration of 235UF6 to the required level for the nuclear reactor. The principle behind this process, known as Graham's law, is explained in the chapter on gases. The enriched UF6 gas is collected and cooled until it solidifies, and then it is taken to a fabrication facility where it is transformed into fuel assemblies. Each fuel assembly contains fuel rods that house numerous small, ceramic-encased fuel pellets made from enriched uranium, typically UO2. Modern nuclear reactors can have up to 10 million fuel pellets, each of which contains as much energy as nearly a ton of coal or 150 gallons of oil.
Nuclear Moderators
Nuclear reactions generate fast-moving neutrons that are initially too speedy to induce fission (see Figure 4). To enable fission, these neutrons must first be slowed down to a manageable speed, allowing them to be absorbed by the fuel and initiate additional nuclear reactions. A nuclear moderator is a substance employed to decelerate the neutrons to a sufficiently low speed for fission to occur. In earlier reactors, high-purity graphite was used as a moderator. However, modern reactors in the United States exclusively utilize heavy water  or light water (ordinary H2O) as moderators. In contrast, reactors in other countries may employ alternative materials like carbon dioxide, beryllium, or graphite for this purpose.
or light water (ordinary H2O) as moderators. In contrast, reactors in other countries may employ alternative materials like carbon dioxide, beryllium, or graphite for this purpose.
Reactor Coolants
A coolant in a nuclear reactor serves the purpose of transferring the heat generated by the fission reaction to an external boiler and turbine, where it is converted into electricity. To prevent the transfer of radioactivity from the reactor to the primary coolant loop, a common practice involves using two overlapping coolant loops. In the United States, water is the coolant of choice in all nuclear power plants. However, alternative coolants such as molten sodium, lead, a lead-bismuth mixture, or molten salts can also be employed.
Control Rods
Control rods are utilized in nuclear reactors to regulate the fission rate of the nuclear fuel. Their purpose is to adjust the number of slow neutrons present in order to maintain a safe level of chain reaction. These control rods are typically composed of elements such as boron, cadmium, hafnium, or other neutron-absorbing materials. An example is boron-10, which absorbs neutrons through a reaction that generates lithium-7 and alpha particles: When control rod assemblies are inserted into the fuel element within the reactor core, they absorb a greater proportion of the slower neutrons, leading to a reduction in the fission reaction rate and a decrease in power generation. On the other hand, by removing the control rods, fewer neutrons are absorbed, resulting in an increase in the fission rate and energy production. In the event of an emergency, the chain reaction can be halted by fully inserting all of the control rods into the nuclear core, effectively shutting it down, positioning them between the fuel rods.
When control rod assemblies are inserted into the fuel element within the reactor core, they absorb a greater proportion of the slower neutrons, leading to a reduction in the fission reaction rate and a decrease in power generation. On the other hand, by removing the control rods, fewer neutrons are absorbed, resulting in an increase in the fission rate and energy production. In the event of an emergency, the chain reaction can be halted by fully inserting all of the control rods into the nuclear core, effectively shutting it down, positioning them between the fuel rods.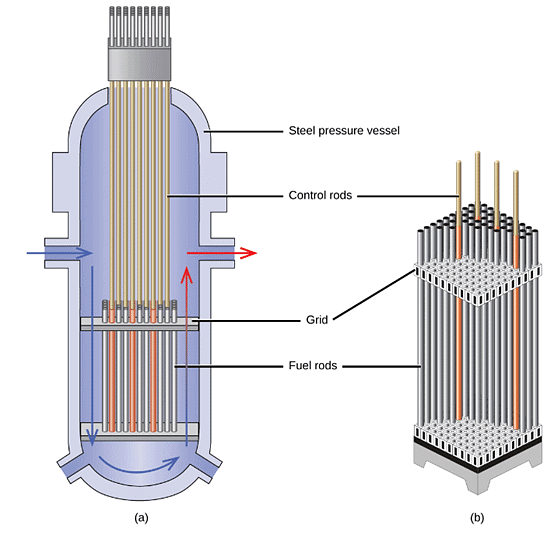
Figure 7: The nuclear reactor core shown in (a) contains the fuel and control rod assembly shown in (b).
Shield and Containment System
During its operation, a nuclear reactor produces neutrons and other radiation. Even when shut down, the decay products are radioactive. In addition, an operating reactor is thermally very hot, and high pressures result from the circulation of water or another coolant through it. Thus, a reactor must withstand high temperatures and pressures, and must protect operating personnel from the radiation. Reactors are equipped with a containment system (or shield) that consists of three parts:
- The reactor vessel, a steel shell that is 3–20-centimeters thick and, with the moderator, absorbs much of the radiation produced by the reactor
- A main shield of 1–3 meters of high-density concrete
- A personnel shield of lighter materials that protects operators from γ rays and X-rays
In addition, reactors are often covered with a steel or concrete dome that is designed to contain any radioactive materials might be released by a reactor accident.
The design of nuclear power plants ensures that they are incapable of reaching a supercritical mass of fissionable material, thereby preventing the occurrence of a nuclear explosion. However, historical events have demonstrated that system failures and safety breaches can result in devastating accidents, such as chemical explosions and nuclear meltdowns (which involve severe damage to the reactor core due to overheating). The upcoming Chemistry in Everyday Life feature delves into three well-known incidents involving nuclear meltdowns.
Nuclear Accidents
The importance of cooling and containment are amply illustrated by three major accidents that occurred with the nuclear reactors at nuclear power generating stations in the United States (Three Mile Island), the former Soviet Union (Chernobyl), and Japan (Fukushima).
During March 1979, the cooling system of the Unit 2 reactor at Three Mile Island Nuclear Generating Station in Pennsylvania experienced a malfunction, causing the cooling water to leak from the reactor and onto the containment building floor. Following the shutdown of the nuclear reactor, the absence of functioning pumps resulted in excessive heat generated by radioactive decay in the initial days. As a consequence, the core temperature rose to a minimum of 2200 °C, leading to the partial melting of the upper portion of the core. Additionally, the zirconium alloy cladding of the fuel rods reacted with steam, resulting in the production of hydrogen. The confinement building experienced a buildup of hydrogen gas, raising concerns about the potential explosion of the hydrogen-air mixture within the structure. As a precautionary measure, both hydrogen gas and radioactive gases, mainly krypton and xenon, were released from the building. Within a week, the circulation of cooling water was reinstated, initiating the cooling process of the reactor core. As a result of the incident, the plant remained shut down for approximately 10 years to undergo the necessary cleanup procedures.
The confinement building experienced a buildup of hydrogen gas, raising concerns about the potential explosion of the hydrogen-air mixture within the structure. As a precautionary measure, both hydrogen gas and radioactive gases, mainly krypton and xenon, were released from the building. Within a week, the circulation of cooling water was reinstated, initiating the cooling process of the reactor core. As a result of the incident, the plant remained shut down for approximately 10 years to undergo the necessary cleanup procedures.
While achieving a zero discharge of radioactive substances is preferable, the release of radioactive krypton and xenon, as observed in the Three Mile Island incident, is considered relatively acceptable. These gases have the characteristic of quickly dispersing in the atmosphere, minimizing the creation of highly radioactive zones. Additionally, being noble gases, they do not become part of the food chain as they are not incorporated into plant or animal matter. Importantly, none of the more substantial elements from the reactor core were released into the environment, rendering any cleanup efforts outside the containment building unnecessary (refer to Figure 8).
 Figure 8: (a) In this 2010 photo of Three Mile Island, the remaining structures from the damaged Unit 2 reactor are seen on the left, whereas the separate Unit 1 reactor, unaffected by the accident, continues generating power to this day (right).
Figure 8: (a) In this 2010 photo of Three Mile Island, the remaining structures from the damaged Unit 2 reactor are seen on the left, whereas the separate Unit 1 reactor, unaffected by the accident, continues generating power to this day (right).
(b) President Jimmy Carter visited the Unit 2 control room a few days after the accident in 1979.
In April 1986, a significant nuclear accident took place at the Chernobyl Nuclear Power Plant in Ukraine, which was part of the former Soviet Union at the time. The incident occurred when one of the reactors, operating at low power as part of an unauthorized experiment with safety measures disabled, became unstable. The chain reaction within the reactor spiraled out of control, surpassing its intended limits. The resulting steam pressure in the reactor reached levels 100 to 500 times higher than the normal operating pressure, causing the reactor to rupture. Due to the absence of a containment building, a substantial amount of radioactive material was released, exacerbated by the ignition and burning of the graphite (carbon) moderator in the core. Although the fire was eventually contained, more than 200 plant workers and firefighters suffered from acute radiation sickness, with at least 32 fatalities directly attributed to radiation exposure. It is estimated that approximately 4,000 additional deaths will occur among emergency workers and former Chernobyl residents due to radiation-induced cancer and leukemia. The reactor has been encased in a structure of steel and concrete, known as the sarcophagus, although it is currently deteriorating. Even after nearly 30 years, the area still experiences significant radiation issues, and Chernobyl remains predominantly uninhabitable.
In 2011, the Fukushima Daiichi Nuclear Power Plant in Japan suffered extensive damage due to a powerful 9.0-magnitude earthquake and subsequent tsunami. At the time of the disaster, three reactors were operational and automatically shut down as a safety measure. Emergency generators were activated to provide electricity for essential systems and coolants. However, the tsunami swiftly inundated the emergency generators, cutting off power supply to the pumps responsible for circulating coolant water throughout the reactors. As a result, high-temperature steam within the reactors reacted with the zirconium alloy, leading to the generation of hydrogen gas. The hydrogen gas escaped into the containment building and mixed with air, resulting in an explosion. Deliberate venting was conducted to reduce hydrogen pressure, coolant water was intentionally discharged into the sea, and unintentional or uncontrolled events occurred, leading to the release of radioactive material from the containment vessels.
The evacuation zone surrounding the impaired Fukushima Daiichi Nuclear Power Plant covered a distance of 12.4 miles, leading to the evacuation of approximately 200,000 individuals from the affected area. As a consequence of the incident, all 48 nuclear power plants in Japan were subsequently halted and have remained inactive as of December 2014. Following the disaster, public sentiment has undergone a notable shift, transitioning from predominantly supportive of nuclear power plants to predominantly opposed. As a result, the resumption of Japan's atomic energy program has encountered significant obstacles, resulting in its current stagnation (refer to Figure 9).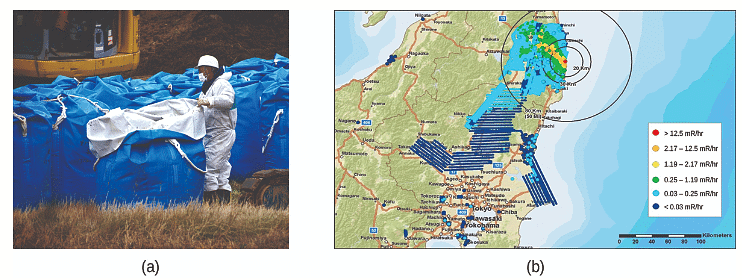
Figure 9: (a) After the accident, contaminated waste had to be removed, and
(b) an evacuation zone was set up around the plant in areas that received heavy doses of radioactive fallout.
The energy generated by a reactor utilizing enriched uranium is a result of the fission process involving both uranium and plutonium. As previously explained, plutonium is formed when neutrons combine with uranium within the fuel. In any nuclear reactor, only a small fraction, approximately 0.1%, of the fuel's mass is converted into energy. The remaining 99.9% remains in the fuel rods as fission products and unused fuel. All fission products have the ability to absorb neutrons, and after a period of several months to a few years, depending on the reactor, it becomes necessary to replace the fuel rods in order to remove these fission products. Otherwise, the concentration of these fission products would gradually increase, leading to a greater absorption of neutrons until the reactor becomes inoperable.
Spent fuel rods encompass a range of components, including unstable nuclei with atomic numbers spanning from 25 to 60, transuranium elements like plutonium and americium, as well as unreacted uranium isotopes. These constituents contribute to the high level of radioactivity found in spent fuel. The radioactive decay of long-lived isotopes within the fuel rods takes thousands of years to reach a safe level. The future prospects of nuclear reactors as a substantial energy source in the United States hinge on the development of a politically and scientifically viable technique for processing and storing the components of spent fuel rods in a satisfactory manner.
|
110 videos|124 docs|114 tests
|

|
Explore Courses for ACT exam
|

|
















