Fluid Mosaic Model | Biology for MCAT PDF Download
| Table of contents |

|
| Introduction |

|
| What’s it made up of? |

|
| What makes the cell membrane fluid? |

|
| What can go through the cell membrane? |

|
Introduction
Contrary to its seemingly chaotic composition, the human body is intricately organized. Within individual cells, essential elements such as liquid nutrients, cellular machinery, and genetic instructions are enclosed by a double layer of lipids, known as the cell membrane.
The primary function of the cell membrane is to maintain the structural integrity of the cell and shield it from the external environment. Additionally, the cell membrane plays a vital role in controlling the passage of substances into and out of the cell. It carefully regulates the entry of nutrients and ions to prevent excessive loss or intake. Furthermore, the cell membrane serves as a protective barrier, effectively barring harmful substances from entering the cell.
What’s it made up of?
The main constituents of the cell membrane consist of three essential components: 1. Phospholipids 2. Cholesterol 3. Proteins.
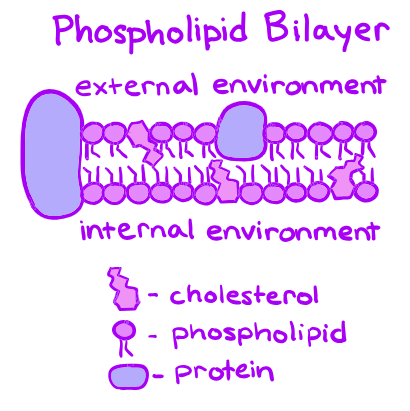
- Phospholipids: Phospholipids, the main components of the cell membrane, consist of two crucial parts: the hydrophilic head and the hydrophobic tails. The head is made of a phosphate molecule that attracts water, while the tails are composed of fatty acids that repel water. Due to these opposing properties, phospholipids arrange themselves into a double layer within the cell membrane, with the heads facing the aqueous environment and the tails hiding between the layers. This self-assembly characteristic ensures the stability and integrity of the membrane.
- Cholesterol: Cholesterol, a type of steroid, plays a role in regulating the movement of molecules in and out of the cell. It is an essential component of the cell membrane and contributes to its structure and function. The specific functions of cholesterol in the cell membrane will be discussed in more detail later.
- Proteins: Proteins are another important component of the cell membrane, and they can be classified into two categories. Integral proteins are embedded within the phospholipid bilayer, extending across its width. These proteins play a crucial role in transporting larger molecules, such as glucose, across the membrane. They have distinct regions known as polar and nonpolar regions, which correspond to the polarity of the phospholipid bilayer.
On the other hand, peripheral proteins are not embedded in the membrane but are instead attached to the ends of integral proteins or found on the inner or outer surface of the membrane. They assist in various cellular functions, including transport and communication.
What makes the cell membrane fluid?
The cell membrane is described by scientists using the fluid mosaic model, which illustrates its appearance and function as a collection of various molecules distributed throughout the membrane. Zooming in on the cell membrane would reveal a pattern of different types of molecules assembled together, resembling a mosaic. These molecules constantly move in a fluid manner within the membrane, comparable to icebergs floating in the ocean. The movement of these molecules prevents the formation of a completely impenetrable barrier.
Cell membrane fluidity is influenced by three main factors:
- Temperature: Temperature affects the movement and proximity of phospholipids within the membrane. Lower temperatures cause the phospholipids to be closer together, while higher temperatures result in increased separation.
- Cholesterol: Cholesterol molecules are randomly distributed within the phospholipid bilayer and help maintain its fluidity under different environmental conditions. Cholesterol plays a role in holding the phospholipids together, preventing excessive separation or compacting. Without cholesterol, phospholipids would come closer together in cold conditions, hindering the entry of small molecules. Conversely, they would separate from each other, creating gaps in the absence of cholesterol.
- Saturated and unsaturated fatty acids: Fatty acids form the tails of phospholipids. Saturated fatty acids consist of carbon chains with only single bonds, resulting in straight chains that can tightly pack together. Unsaturated fatty acids, on the other hand, have double bonds between some carbon atoms, introducing kinks in the chains that hinder tight packing.
- The presence of kinks in unsaturated fatty acids influences membrane fluidity by increasing the space between phospholipids, making it more difficult for the membrane to freeze at lower temperatures. Additionally, the increased space allows certain small molecules like carbon dioxide (CO2) and oxygen (O2) to easily cross the membrane.
Overall, the fluid mosaic model and the factors affecting cell membrane fluidity highlight the dynamic nature of the membrane, allowing for essential functions such as selective permeability and fluid movement of molecules.
What can go through the cell membrane?
Phospholipids exhibit both attraction to each other and constant motion, causing them to bounce off one another. Despite their fluidity, the spaces within the membrane are extremely small, making it an effective barrier. This, along with the assistance of proteins in transport, is why cell membranes are referred to as semi-permeable.
In the cellular environment, molecules can be categorized into five broad groups based on their behavior with water. This distinction is often based on their hydrophilic (water-loving) or hydrophobic (water-fearing) nature, which determines their ability to form bonds with water and other molecules. These categories are as follows:
- Small, nonpolar molecules (e.g., oxygen and carbon dioxide): These molecules can easily pass through the lipid bilayer by squeezing through the phospholipid layers. They do not require transport proteins and can diffuse rapidly.
- Small, polar molecules (e.g., water): Although these molecules can pass through the lipid bilayer without the aid of proteins, it is a relatively slower process compared to small, nonpolar molecules. Water molecules, for example, encounter difficulty crossing the hydrophobic tails of the phospholipid bilayer.
- Large, nonpolar molecules (e.g., carbon rings): While these larger nonpolar molecules can pass through the membrane, the process is slow and may require additional time.
- Large, polar molecules (e.g., simple sugars like glucose): The size and charge of large polar molecules make it challenging for them to pass through the nonpolar region of the phospholipid membrane without the assistance of transport proteins.
- Ions (e.g., Na+): Ions, due to their charge, face similar challenges in crossing the nonpolar region of the phospholipid membrane without the aid of transport proteins.
The semi-permeability of the cell membrane allows for the selective transport of molecules, ensuring the entry and exit of specific substances while maintaining the internal environment of the cell.
|
233 videos|16 docs|32 tests
|




















