Heating and Cooling Curves | Chemistry for EmSAT Achieve PDF Download
Heating Curves
What occurs to the temperature of a block of ice when a Bunsen burner is placed underneath it? The intuitive expectation would be for the temperature to increase steadily, but that is not the case. The temperature-time relationship is depicted by a heating curve graph. Let's examine the heating curve specifically for water.
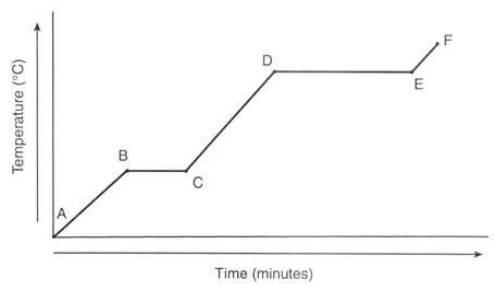
Note that, in general, the temperature increases with prolonged heating. However, there are two distinct flat sections (segments BC and DE) in the graph. These segments occur during a change of state and are commonly referred to as phase changes or plateaus. The first change of state (segment BC) corresponds to the process of melting, where a substance transitions from a solid to a liquid. During this phase change, the temperature remains constant. For water, this temperature is 0°C, known as its melting point. Throughout segment BC, both solid and liquid phases coexist in varying proportions, ranging from 100% solid to 100% liquid.
The second change of state (segment DE) represents boiling, the conversion of a liquid into a gas. Similar to melting, the temperature remains constant during this phase change. For water, the temperature at this plateau is 100°C, which is its boiling point. Along segment DE, both liquid and gas phases coexist in different ratios, ranging from 100% liquid to 100% gas. It's important to note that different substances have distinct melting and boiling points, but their heating curves exhibit similar shapes.
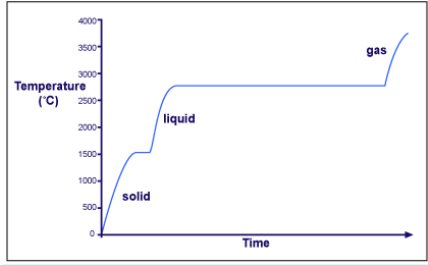
For instance, let's consider the heating curve for iron, a metal that melts at 1538°C and boils at 2861°C.
Cooling Curves
Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. They show how the temperature changes as a substance is cooled down. Just like heating curves, cooling curves have horizontal flat parts where the state changes from gas to liquid, or from liquid to solid. These are mirror images of the heating curve. You will use lauric acid in a school lab to make your own cooling curve. Lauric acid has a melting point of about 45°C and is easily melted in a test tube placed in a beaker of hot water. The temperature can be followed using a thermometer or temperature probe connected to a data logger. The liquid may be cooled by putting the boiling tube in a beaker of cold water or just leaving it in the air.
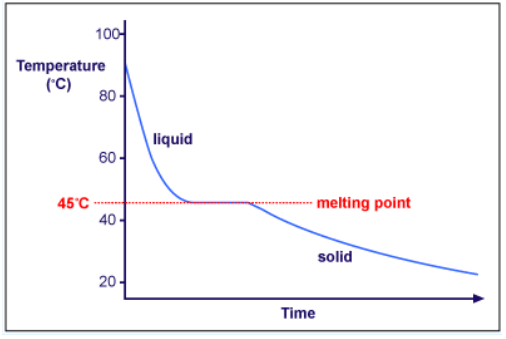
Note- The melting and freezing occur at the same temperature. During freezing, energy is removed and during melting, energy is absorbed.
Energy Changes
- Temperature serves as an indicator of the average kinetic energy of a substance. Therefore, any alteration in temperature implies a modification in kinetic energy. The diagonal line segments depicted in heating or cooling curves signify both a temperature change and a corresponding shift in kinetic energy. Within these segments, the substance remains in a single state of matter and either experiences an increase or decrease in temperature.
- On the other hand, during the horizontal line segments, there is no fluctuation in temperature, indicating that the kinetic energy remains constant. Nevertheless, all the absorbed or released energy is associated with alterations in potential energy.
|
195 videos|213 docs|157 tests
|

|
Explore Courses for EmSAT Achieve exam
|

|

















