What is Corrosion? | Chemistry for EmSAT Achieve PDF Download
| Table of contents |

|
| Introduction |

|
| Rate of Corrosion |

|
| Types of Corrosion |

|
| Corrosion Examples, Reactions and Effects |

|
| Effects |

|
Introduction
- Corrosion refers to the natural process in which pure metals react with substances like water or air, resulting in the transformation of the metal into undesirable substances. This process leads to damage and disintegration of the metal, starting from the exposed portion and spreading throughout its entire bulk.
- Corrosion is generally considered undesirable as it negatively affects the desirable properties of metals. For instance, iron is known for its good tensile strength and rigidity, especially when alloyed with other elements. However, when it undergoes rusting, iron objects become brittle, flaky, and structurally unsound. Despite this, corrosion is a diffusion-controlled process that primarily occurs on exposed surfaces. Therefore, efforts are sometimes made to reduce the activity of exposed surfaces and enhance the corrosion resistance of materials. Passivation and chromate conversion are commonly employed processes for this purpose. However, some corrosion mechanisms may not be readily visible and are difficult to predict.
- Corrosion can be classified as an electrochemical process since it typically involves redox reactions between the metal and atmospheric agents like water, oxygen, sulphur dioxide, and others.
Do All Metals Corrode?
- Not all metals corrode in the same manner. Metals higher in the reactivity series, such as iron and zinc, are more prone to corrosion, while metals lower in the reactivity series, like gold, platinum, and palladium, are less susceptible to corrosion. This can be explained by the fact that corrosion involves the oxidation of metals. As we move down the reactivity series, the tendency of metals to undergo oxidation decreases (their oxidation potentials are lower).
- Aluminium is a unique metal that does not undergo corrosion like other metals, despite its reactivity. The reason behind this lies in the fact that aluminium naturally forms a layer of aluminium oxide on its surface. This protective layer acts as a barrier, preventing further corrosion of the metal.
Factors Affecting Corrosion
- The exposure of metals to air containing gases such as CO2, SO2, SO3, etc.
- The exposure of metals to moisture, especially saltwater, which accelerates corrosion.
- The presence of impurities like salt (e.g., NaCl).
- Temperature: Corrosion rates increase with higher temperatures.
- The nature of the first layer of oxide formed: Some oxides, like Al2O3, create a protective layer that inhibits further corrosion, while others, like rust, easily crumble and expose the underlying metal.
- The presence of acid in the atmosphere, as acids can expedite the corrosion process.
Rate of Corrosion
The Deal-Grove model is commonly employed to explain the formation of oxide layers, allowing for prediction and control of oxide layer formation in various scenarios. Additionally, the weight loss method is utilized to measure corrosion. This method involves exposing a clean, weighed piece of metal or alloy to a corrosive environment for a specific duration. Afterward, the corrosion products are removed through a cleaning process, and the piece is reweighed to determine the weight loss.
The rate of corrosion (R) is calculated as:
R = kW/pAt
Where,
k = constant,
W = weight loss of the metal in time t,
A = surface area of the metal exposed,
ρ is the density of the metal (in g/cm³).
Types of Corrosion
- Crevice Corrosion: Crevice corrosion occurs when there is a difference in ionic concentration between two local areas of a metal. It often happens in confined spaces such as gaskets, undersurfaces of washers, and bolt heads. Aluminum alloys and stainless steels are susceptible to crevice corrosion due to the formation of a differential aeration cell inside the crevices.
- Stress Corrosion Cracking: Stress corrosion cracking (SCC) refers to the cracking of a metal caused by a corrosive environment and the applied tensile stress. It commonly occurs at high temperatures, such as the stress corrosion cracking of austenitic stainless steel in a chloride solution.
- Intergranular Corrosion: Intergranular corrosion happens due to the presence of impurities at the grain boundaries of a metal alloy formed during solidification. It can also occur due to the depletion or enrichment of the alloy at these grain boundaries. Aluminum-based alloys are susceptible to intergranular corrosion.
- Galvanic Corrosion: Galvanic corrosion occurs when two dissimilar metals are in electrical contact in an electrolytic environment. One metal degrades at the joint or junction. An example is the corrosion that occurs when copper comes in contact with steel in a saltwater environment.
- Pitting Corrosion: Pitting corrosion is an unpredictable and dangerous form of corrosion. It occurs at local points, forming corrosion cells surrounded by the metal's normal surface. These pits continue to grow and can lead to structural failure if not addressed. Pitting corrosion can initiate from a water droplet on a steel surface.
- Uniform Corrosion: Uniform corrosion is the most common form of corrosion, where the entire surface of the metal is attacked by the surrounding atmosphere. The extent of corrosion is easily noticeable, but it has a relatively low impact on material performance.
- Hydrogen Grooving: Hydrogen grooving is the formation of grooves on piping caused by the interaction of a corrosive agent, corroded pipe constituents, and hydrogen gas bubbles. These bubbles remove the protective coating when they come into contact with the material.
- Metal Dusting; Metal dusting is a corrosive process that occurs when certain materials are exposed to environments with high carbon activities. It leads to the disintegration of the metal into metal powder due to the deposition of a graphite layer and the formation of meta-stable M3C species.
- Microbial Corrosion: Microbial corrosion, also known as microbiologically influenced corrosion (MIC), is caused by microorganisms. It affects both metallic and non-metallic materials in the presence or absence of oxygen. Chemoautotrophs are common microorganisms involved in this type of corrosion.
- High-temperature Corrosion: High-temperature corrosion refers to the corrosion of materials, particularly metals, due to exposure to elevated temperatures. The chemical deterioration occurs in the presence of gases such as oxygen, sulphur, or other compounds capable of oxidizing the metals. Materials used in car engines, for example, need to withstand high temperatures and resist corrosive combustion byproducts.
Corrosion Examples, Reactions and Effects
Here are some common instances of corrosion primarily observed in metals:
1. Copper Corrosion: When copper metal is exposed to the environment, it undergoes a reaction with atmospheric oxygen, resulting in the formation of red-colored copper (I) oxide:
2Cu(s) + ½ O2(g) → Cu2O(s)
Subsequently, Cu2O oxidizes further to form black-colored CuO:
Cu2O(s) + ½ O2(g) → 2CuO(s)
CuO reacts with CO2, SO3, and H2O present in the atmosphere, leading to the formation of blue-colored Cu2(OH)2(s) (Malachite) and green-colored Cu4SO4(OH)6(s) (Brochantite). This is why we observe copper turning bluish-green in color. The Statue of Liberty serves as a well-known example, showcasing the transformation of its copper coating to a blue-green hue.
2. Silver Tarnishing
Silver reacts with sulfur and sulfur compounds in the air, resulting in the formation of black-colored silver sulfide (Ag2S). This occurs when exposed silver reacts with H2S(g) present in the atmosphere, typically originating from certain industrial processes:
2Ag(s) + H2S(g) → Ag2S(s) + H+2+(g)
3. Corrosion of Iron (Rusting)
Rusting of iron is the most commonly observed form of corrosion, transpiring when iron comes into contact with air or water. The process can be described as a typical electrochemical cell reaction. Please refer to the provided diagram for further details.
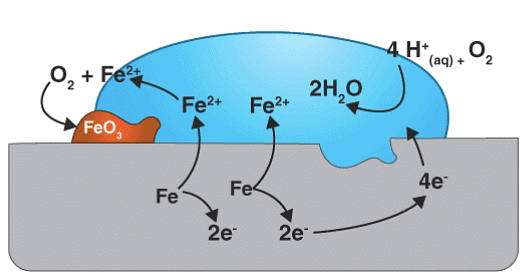
Here, metal iron loses electrons and gets converted to Fe{aq}2+ (this could be considered as the anode position). The electrons lost will move to the other side, where they combine with H+ ions. H+ ions are released either by H2O or by H2CO3 present in the atmosphere (this could be considered as the cathode position).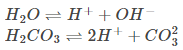
The Hydrogen, thus formed by the reaction of H+ and electrons, react with oxygen to form H2O.
Anode reaction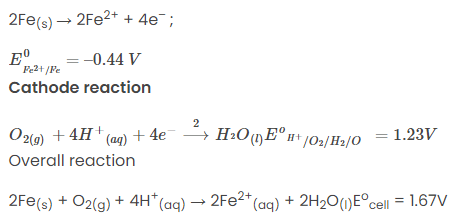
The Fe2+ ions formed at the anode react with oxygen in the atmosphere, thereby getting oxidised to Fe3+ and forming Fe2O3, which comes out in the hydrated form as Fe2O3.xH2O
Fe2+ + 3O2 → 2Fe2O3
Fe2O3 + xH2O → Fe2O3. xH2O (rust)
Other examples include,
- Corrosion of Zinc when it reacts with oxygen and HCl to form white-coloured ZnCl2.
- Corrosion of Tin to form black-coloured Na2[Sn(OH)2].
Effects
Corrosion can have various impacts on a wide range of entities, primarily leading to the wastage of natural resources. Furthermore, it can give rise to hazardous situations such as weakened and unstable building structures, accidents caused by corroded components, and other undesirable failures like cracked pipelines, bridge collapses, vehicle crashes, or similar catastrophes. It is therefore crucial to diligently inspect and prevent corrosion in order to mitigate these risks.
Prevention of Corrosion
Preventing corrosion is paramount to avoid substantial losses. The majority of structures and objects we encounter and utilize are composed of metals. This includes bridges, automobiles, machinery, household items like window grills, doors, and railway lines. While this poses a significant concern, several treatments are employed to impede or prevent corrosion damage to metallic objects, particularly those frequently exposed to adverse conditions such as weather, saltwater, acids, or hostile environments. Some popular methods for corrosion prevention include:
- Electroplating
- Galvanization
- Anodization
- Passivation
- Biofilm Coatings
- Anti-Corrosion Protective Coatings
- Painting and Greasing
- Utilization of Corrosion Inhibitors or Drying Agents
- Regular Cleaning of Metal Surfaces
|
191 videos|265 docs|160 tests
|














