Carboxylic Acid Reactions: Understanding the Transformations and Mechanisms | Organic Chemistry for MCAT PDF Download
| Table of contents |

|
| Introduction |

|
| Nucleophilic Substitution in Carboxylic Acids |

|
| Carboxylic Acid Derivatives Overview |

|
| Reactivity of Carboxylic Acid Derivatives |

|
Introduction
Carboxylic acids, an important class of organic compounds, are characterized by the presence of a carboxyl group (COOH) in their structure. In this article, we will explore the various reactions of carboxylic acids and their derivatives, shedding light on their mechanisms and applications. Understanding these reactions is essential for grasping the fundamental principles of organic chemistry.

Nucleophilic Substitution in Carboxylic Acids
Carboxylic acids undergo nucleophilic substitution reactions, where a nucleophile (Nu) replaces the hydroxyl group (-OH). The polarized nature of the carbonyl group (C=O) facilitates these reactions. The oxygen atom, being more electronegative, attracts electron density and imparts a partial negative charge (δ-) to itself, while the carbon atom develops a partial positive charge (δ+). Notably, in the presence of a strong electrophile, the partially negatively charged carbonyl oxygen can also act as a nucleophile.
Carboxylic Acid Derivatives Overview
Carboxylic acid derivatives are compounds formed by replacing the -OH group of carboxylic acids with various functional groups. The major derivatives include acyl halides, acid anhydrides, esters, and amides. Let's explore their common properties and reactions:
Acid Chloride (ROCl)
Acid chlorides are highly reactive derivatives of carboxylic acids. They are synthesized by reacting carboxylic acids with reagents such as thionyl chloride (SOCl₂), PCl₃, or PCl₅. The mechanism involves the attack of the nucleophilic oxygen of the carboxylic acid on the electrophilic sulfur atom of the reagent, leading to the formation of an intermediate and subsequent conversion to the acyl chloride.

Ester (RCOOR')
Esters are formed by the reaction of carboxylic acids with alcohols. Fischer esterification is the acid-catalyzed process that results in the formation of esters. Notably, esters are important components of fats, oils, and fragrances.

Thioester (RCOSR')
Thioesters are derived when carboxylic acids react with thiols (RSH) in the presence of an acid catalyst. Thioesters play significant roles in biochemical processes, with acetyl CoA being a well-known example.

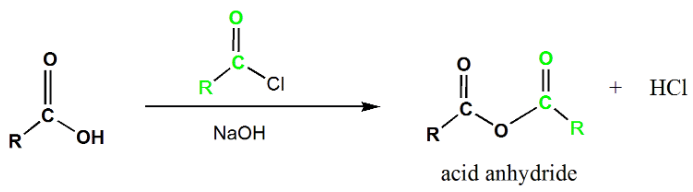
Acid Anhydride
Acid anhydrides contain two acyl groups bonded to the same oxygen atom. They are synthesized by the reaction of carboxylic acids with acid chlorides in the presence of a base. The mechanism involves the addition-elimination process, with the base abstracting a proton from the carboxylic acid and subsequent elimination of chloride ion to yield the acid anhydride.
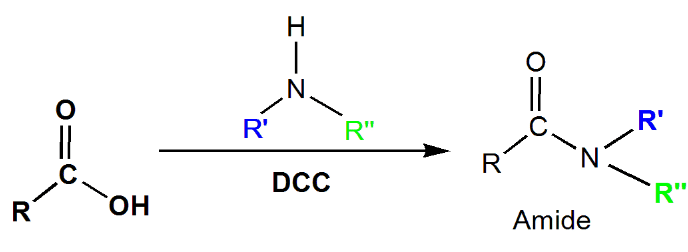
Amide
Direct conversion of carboxylic acids to amides is challenging due to the basic nature of amines, which tend to convert carboxylic acids into carboxylate ions. Dicyclohexylcarbodiimide (DCC) is commonly used as a reagent to drive the reaction, facilitating the substitution of the carboxylate ion with an amine to form the desired amide product.
Reactivity of Carboxylic Acid Derivatives
The reactivity of carboxylic acid derivatives in nucleophilic substitution reactions is influenced by the substituent (X) attached to the acyl group (R-C=O). Electron-donating groups reduce the electrophilicity of the carbonyl group, making the derivative less reactive to nucleophilic substitution.
Conclusion
Understanding the reactions of carboxylic acids and their derivatives is crucial for organic chemistry. The ability to transform carboxylic acids into various derivatives widens the scope of their applications in synthesis and biochemistry. By comprehending the mechanisms and reactivity patterns, aspiring chemists can navigate the complexities of organic reactions with confidence.
|
140 videos|5 docs|15 tests
|

|
Explore Courses for MCAT exam
|

|
















