Endothermic vs. exothermic reactions | General Chemistry for MCAT PDF Download
Introduction
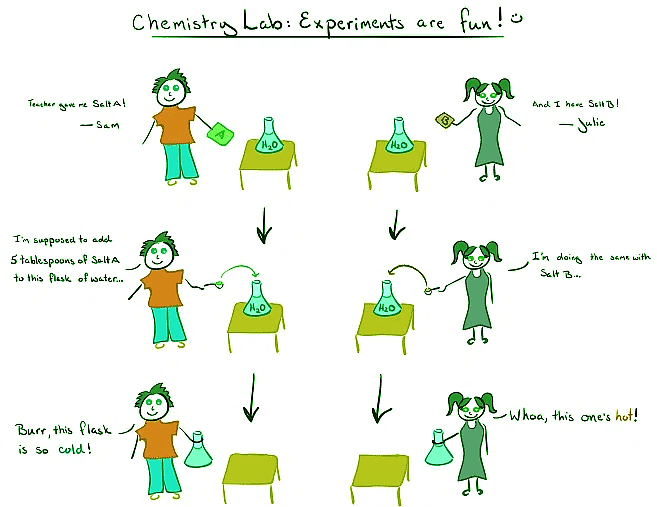
Step into the captivating world of Sam and Julie as they venture into the chemistry lab, fueled by excitement and curiosity. An intriguing encounter with their chemistry teacher leaves them pondering the mysteries of endothermic and exothermic reactions. Join us as we dive deep into the realm of these fascinating chemical phenomena and unravel their secrets.
Understanding the Temperature Dichotomy
Intrigued by their contrasting experiences, Sam and Julie approach their chemistry teacher for an explanation. Sam's flask turned cold upon adding salt to water, while Julie's flask emitted warmth. The teacher unveils the key to this divergence, attributing it to the unique properties of the salts used. Salt A, ammonium nitrate (NH₄NO₃), triggered an endothermic reaction, whereas Salt B, calcium chloride (CaCl₂), incited an exothermic reaction.
Endothermic Reactions Unveiled
To comprehend the disparity between endothermic and exothermic reactions, we must first perceive the reaction mixture—salt plus water—as the system and the flask as the surroundings. When Sam dissolved ammonium nitrate in water, the system absorbed heat from the flask, resulting in a chilly sensation. This exemplifies an endothermic reaction, where energy is absorbed from the surroundings.
Exothermic Reactions Illuminated
On the other hand, Julie's flask emitted warmth when calcium chloride dissolved in water. In this scenario, the system released heat into the surroundings, causing the flask to feel hot. This exemplifies an exothermic reaction, characterized by the release of energy into the environment.
Visual Representation: Endothermic and Exothermic Reactions
To gain a visual understanding, let's examine the representations of the reactions occurring in Sam and Julie's flasks.
Endothermic Reaction:
- Reactants + Heat Energy ⟶ Products
- In this reaction, heat is absorbed, resulting in a temperature drop within the reaction mixture.
Exothermic Reaction:
- Reactants ⟶ Products + Heat Energy
- In this reaction, heat is released, leading to an increase in temperature within the reaction mixture.
Teacher's Insights: Decoding the Temperature Shifts
The chemistry teacher imparts valuable wisdom to Sam and Julie, emphasizing the importance of monitoring the temperature shifts in their surroundings to classify reactions as exothermic or endothermic. In an exothermic process, the immediate surroundings experience a temperature rise due to the released heat. Conversely, an endothermic process absorbs heat, causing the surroundings to cool.
Everyday Encounters with Endothermic and Exothermic Reactions
Now that we comprehend the definitions, let's explore some familiar scenarios and classify them as either endothermic or exothermic reactions.
Endothermic Reactions: Heat Absorbed
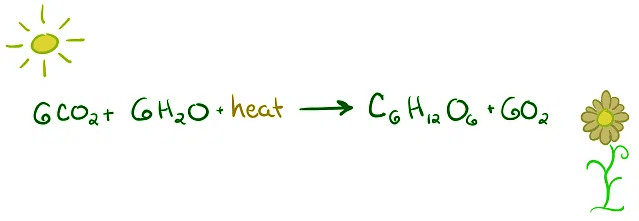
- Photosynthesis: Plants harness sunlight's heat energy to convert carbon dioxide and water into glucose and oxygen.
- Cooking an Egg: The heat energy absorbed from the pan cooks the egg.
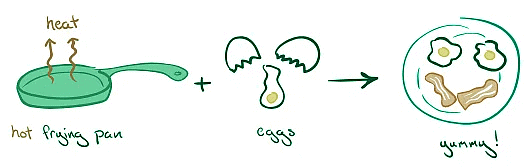
Exothermic Reactions: Heat Released
- Combustion: Carbon-containing compounds burn in the presence of oxygen, producing carbon dioxide, water, and copious amounts of heat. For instance, the combustion of methane (CH₄) can be represented as follows: CH₄ + 2O₂ ⟶ CO₂ + 2H₂O + Heat Energy
- Rain: The condensation of water vapor into raindrops releases energy in the form of heat—an exquisite example of an exothermic process.
Deciphering Heat Exchange in Chemical Reactions
In any chemical reaction, the formation or breaking of chemical bonds plays a pivotal role in heat exchange. A fundamental rule emerges: when chemical bonds form, heat is released, whereas breaking bonds necessitates heat absorption. As molecules inherently strive to remain united, forming bonds between them requires less energy compared to breaking existing bonds. This fundamental disparity leads to the absorption or release of heat, subsequently affecting the surrounding environment.
Delving into Enthalpy: The Energy Change
Enthalpy, denoted as ΔH, characterizes the heat energy change that occurs when reactants transform into products. A positive ΔH signifies heat absorption during the reaction, indicating an endothermic process. Conversely, a negative ΔH represents heat release, signifying an exothermic reaction.
- ΔH = ΣΔH(bonds broken in reactants) - ΣΔH(bonds formed in products)
A Case Study: Calculating Enthalpy Change
To gain practical insight, let's calculate the enthalpy change (ΔH) for a specific reaction:
- H₂(g) + F₂(g) ⟶ 2HF(g)
By referencing the bond energies in kilojoules per mole (kJ/mol) for H₂, F₂, and HF as 436, 158, and 568 kJ/mol respectively, we can analyze this reaction. Breaking one mole of H-H bonds requires 436 kJ, breaking one mole of F-F bonds necessitates 158 kJ, and forming two moles of HF releases 2 × 568 kJ. Applying the equation ΔH = ΣΔH(bonds broken) - ΣΔH(bonds formed), we find ΔH = (436 + 158) - (2 × 568) = -542 kJ. As the overall enthalpy change is negative, this reaction is exothermic, releasing energy in the form of heat.
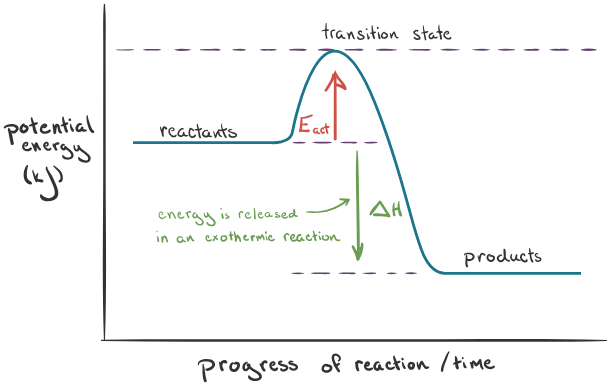
Visualizing Energy Diagrams
Energy diagrams offer a comprehensive representation of chemical reactions. As bonds break and form, an intermediate stage emerges—referred to as the transition state. This transitional phase, exhibiting higher energy levels than the reactants, is incredibly unstable. Activation energy (Eₐct) denotes the minimum energy required to initiate a reaction. An energy diagram displays the relative potential energies of reactants, transition states, and products as the reaction progresses.
Analyzing Endothermic and Exothermic Energy Diagrams
In an endothermic reaction, the reactants reside at lower energy levels than the products, implying greater stability in the initial state. Contrarily, exothermic reactions position the reactants at higher energy levels than the products, indicating enhanced stability from the outset. The energy diagram for an endothermic reaction showcases a rise in potential energy from reactants to products, whereas an exothermic reaction exhibits a descent in potential energy.
Conclusion
Embark on an enchanting journey through the captivating realms of endothermic and exothermic reactions. Unveiling the mysteries behind temperature shifts, bond formations, and energy exchanges, we have delved into the intricate intricacies of these chemical wonders. Armed with newfound knowledge, you can now decipher the diverse landscapes of energy transformations that unfold in the realm of chemistry.
|
164 videos|11 docs|16 tests
|




















