Understanding the Power Behind Everyday Cells | General Chemistry for MCAT PDF Download
Introduction
Electrochemistry plays a vital role in our daily lives, powering the devices we rely on and even governing the intricate processes within our bodies. From the ubiquitous AA batteries that fuel our remote controls to the advanced lithium-ion batteries in our smartphones, electrochemical cells are at the heart of these energy sources. In this article, we will explore the two main types of electrochemical cells: galvanic and electrolytic. By understanding the underlying principles and components of these cells, we can gain insight into their functionality and applications.
Galvanic Cells: Harnessing Spontaneous Reactions for Power
The Basics of Galvanic Cells:
Galvanic cells, also known as voltaic cells, derive their energy from spontaneous redox reactions. These cells are commonly used as sources of direct current (DC) electrical power. A simple galvanic cell consists of two electrodes (an anode and a cathode) immersed in an electrolyte, while more complex versions utilize a salt bridge for increased efficiency.
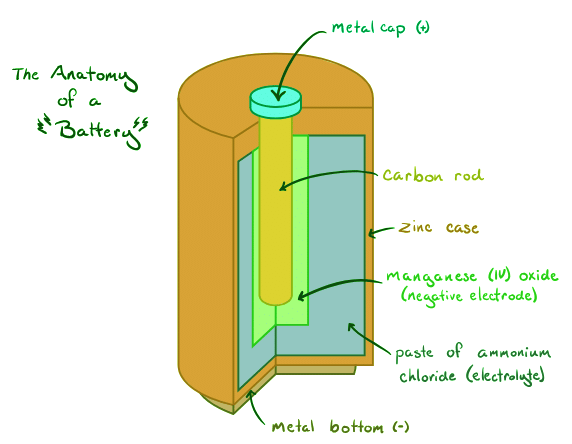
Redox Reactions in Galvanic Cells:
In galvanic cells, oxidation occurs at the anode, while reduction takes place at the cathode. The anode serves as the source of electrons for the current flow, making it the negative terminal of the cell. By following the mnemonic "Red Cat An Ox," we can easily remember the electrode processes involved.
Example: The AA Alkaline Battery:
Let's examine the classic AA alkaline battery as an example of a galvanic cell. The half-reactions involved in this battery can be represented using equations. By determining the reduction potentials for each half-reaction and calculating the overall cell electromotive force (EMF), we can gain insights into the battery's performance.
Electrolytic Cells: Driving Non-Spontaneous Reactions with External Energy
Understanding Electrolytic Cells:
Electrolytic cells, unlike galvanic cells, require an external energy source, such as a DC battery or an AC power source, to drive non-spontaneous reactions. These cells are essential for various industrial processes and the decomposition of compounds into simpler substances.
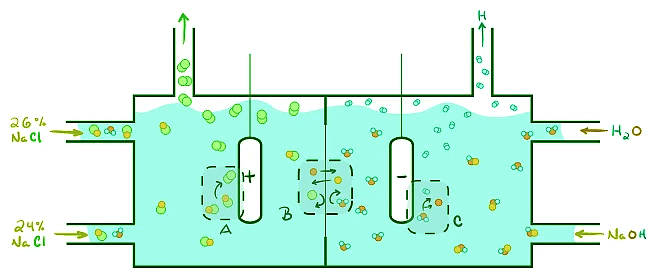
Electrolysis of Sodium Chloride:
One application of electrolytic cells is the production of chlorine or sodium hydroxide. By examining the electrolysis of sodium chloride in aqueous solution, we can understand the different reactions and products involved. The oxidation of chloride ions and reduction of water play crucial roles in this process.
Calculating and Understanding Cell Potentials
Connection between EMF and Gibbs Free Energy:
The standard state electromotive force (EMF) of a cell can be related to the Gibbs free energy change (∆G) through a mathematical equation. Understanding this connection allows us to express energy changes in more thermodynamic terms.
Calculating Gibbs Free Energy:
By using the equation ∆G = -nFE°, where n represents the moles of electrons transferred and F is Faraday's constant, we can calculate the Gibbs free energy change. This value provides insights into the spontaneity of a reaction.
Equilibrium Constant and Concentration Cells
Relating EMF and Equilibrium Constant:
Determining the equilibrium constant (K) allows us to assess the favorability of a reaction. By using the relationship between ∆G° and K, we can calculate the equilibrium constant at standard conditions. Concentration Cells:
Concentration cells generate a small voltage as they strive to reach equilibrium. The Nernst Equation provides a means to calculate the potential developed by a concentration cell. By understanding this equation, we can analyze the role of concentrations in the development of potential in concentration cells.
In a concentration cell, both half-cells contain the same components but with different concentrations. This concentration gradient drives the flow of electrons, generating a voltage. The Nernst Equation allows us to quantify this relationship:
E = E° - (RT / nF) * ln(Q)
where E is the cell potential, E° is the standard cell potential, R is the gas constant, T is the temperature in Kelvin, n is the number of moles of electrons transferred, F is Faraday's constant, and Q is the reaction quotient.
The Nernst Equation shows that the cell potential depends not only on the standard cell potential but also on the concentration of the species involved. As the concentrations change, the cell potential shifts, leading the cell to approach equilibrium. By understanding concentration cells and the Nernst Equation, scientists and engineers can design and optimize electrochemical systems, such as fuel cells and sensors, by manipulating the concentrations of species within the cell.
Applications of Electrochemistry
Batteries and Energy Storage:
Electrochemistry has revolutionized portable power sources. From small alkaline batteries to high-capacity lithium-ion batteries, electrochemical cells provide the energy needed to run devices ranging from everyday electronics to electric vehicles. Ongoing research focuses on improving battery performance, lifespan, and sustainability.
Corrosion Prevention:
Corrosion is a significant issue in various industries, causing damage and financial losses. Electrochemical methods like cathodic protection and electroplating are employed to prevent corrosion. These techniques involve controlling the flow of electrons to protect metal surfaces and ensure their longevity.
Electrolysis and Industrial Processes:
Electrolysis is widely used in industrial processes such as metal extraction, electroplating, and the production of chemicals like chlorine, sodium hydroxide, and hydrogen gas. These processes utilize electrolytic cells to drive non-spontaneous reactions and facilitate the transformation of raw materials into valuable products.
Environmental Applications:
Electrochemistry plays a crucial role in environmental remediation. Electrochemical methods like electrocoagulation and electrooxidation are used to treat wastewater, remove contaminants, and mitigate pollution. Additionally, electrochemical sensors enable rapid and sensitive detection of pollutants and analytes in various environmental samples.
Conclusion
Electrochemistry is a fundamental discipline with widespread applications in our everyday lives. Through galvanic and electrolytic cells, we harness the power of spontaneous and non-spontaneous redox reactions. Understanding the principles, calculations, and applications of electrochemical cells empowers scientists and engineers to develop innovative energy storage solutions, prevent corrosion, drive industrial processes, and address environmental challenges. By further advancing our knowledge in electrochemistry, we can continue to improve technologies and create a more sustainable and efficient future.
|
164 videos|11 docs|16 tests
|














