Class 10 Science Chapter 1 Question Answers - Chemical Reactions and Equations
Short Answer Questions
Q1: Grapes hanging on the plant do not ferment, but after being plucked from the plant can be fermented. Under what conditions do these grapes ferment? Is it a chemical or a physical change?
Ans: When attached to the plants, Grapes are living, and therefore, their immune system prevents fermentation. The microbes can grow in the plucked grapes, which can be fermented under anaerobic conditions. This is a chemical change.
Q2: You are provided with two containers made up of copper and aluminium. You are also provided with dilute HCI, HNO3, ZnCl2 and H2O solutions. In which of the above containers we can keep these solutions?
Ans: The solution of dilute HCI, HNO3, ZnCl2 and H2O can be kept in a container made of copper since copper is a less reactive metal and is placed below the hydrogen in the reactivity series. Hence it does not react with HCI, HNO3, ZnCl2 and H2O. At the same time, aluminium is a highly reactive metal and can react with these solutions. Thus container made of copper is suitable to keep the given solutions.
 Q3: Which among the following are physical or chemical changes?
Q3: Which among the following are physical or chemical changes?
(a) Evaporation of petrol
(b) Burning of Liquefied Petroleum Gas (LPG)
(c) Heating of an iron rod to red hot.
(d) Curdling of milk
(e) Sublimation of solid ammonium chloride
Ans:

(a) Evaporation of petrol is a physical change as it only gets converted from one physical state to another.
(b) Burning of Liquefied Petroleum Gas (LPG) is a chemical change as heating produces carbon dioxide and water.
(c) The heating of an iron rod to red hot is a physical change as heating involves only temperature change.
(d) The curdling of milk is a chemical change as it affects the chemical composition of the milk.
Q4: A substance X, an oxide of a group 2 element, is used intensively in the cement industry. This element is present in bones also. On treatment with water, it forms a solution which turns red litmus blue. Identify X and also write the chemical reactions involved.
Ans: Here, X is calcium oxide.
Calcium oxide is used intensively in the cement industry.
The element present in it (in bones also) is calcium.
On treatment with water, calcium oxide forms a solution of calcium hydroxide [Ca(OH)2], which is an alkali. Hence, it turns red litmus blue.
CaO (s) + H2O (l) → Ca(OH)2 (aq) + Heat

Q5: Write a balanced chemical equation for each following reaction and classify them.
(a) Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution.
(b) A piece of sodium metal is added to absolute ethanol to form sodium ethoxide and hydrogen gas.
(c) Iron (III) oxide on heating with carbon monoxide gas reacts to form solid iron and liberates carbon dioxide gas.
(d) Hydrogen sulphide gas reacts with oxygen gas to form solid sulphur and liquid water
Ans:
(a ) Pb(CH3COO)2 + 2 HCl → PbCl2 + 2 CH3COOH
It is a double displacement reaction.
(b ) 2 Na + 2 C2H5OH → 2 C2H5ONa+ H2
It is a displacement or a redox reaction.
 (c ) Fe2O3 + 3 CO → 2 Fe + 3 CO2
(c ) Fe2O3 + 3 CO → 2 Fe + 3 CO2
It is a redox reaction.
(d ) 2 H2S + O2 → 2 S + 2 H2O
It is a redox reaction.
Q6: A magnesium ribbon is burnt in oxygen to give a white compound X accompanied by light emission. If the burning ribbon is now placed in an atmosphere of nitrogen, it continues to burn and forms a compound Y.
(a) Write the chemical formulae of X and Y.
(b) Write a balanced chemical equation when X is dissolved in water.
Ans:
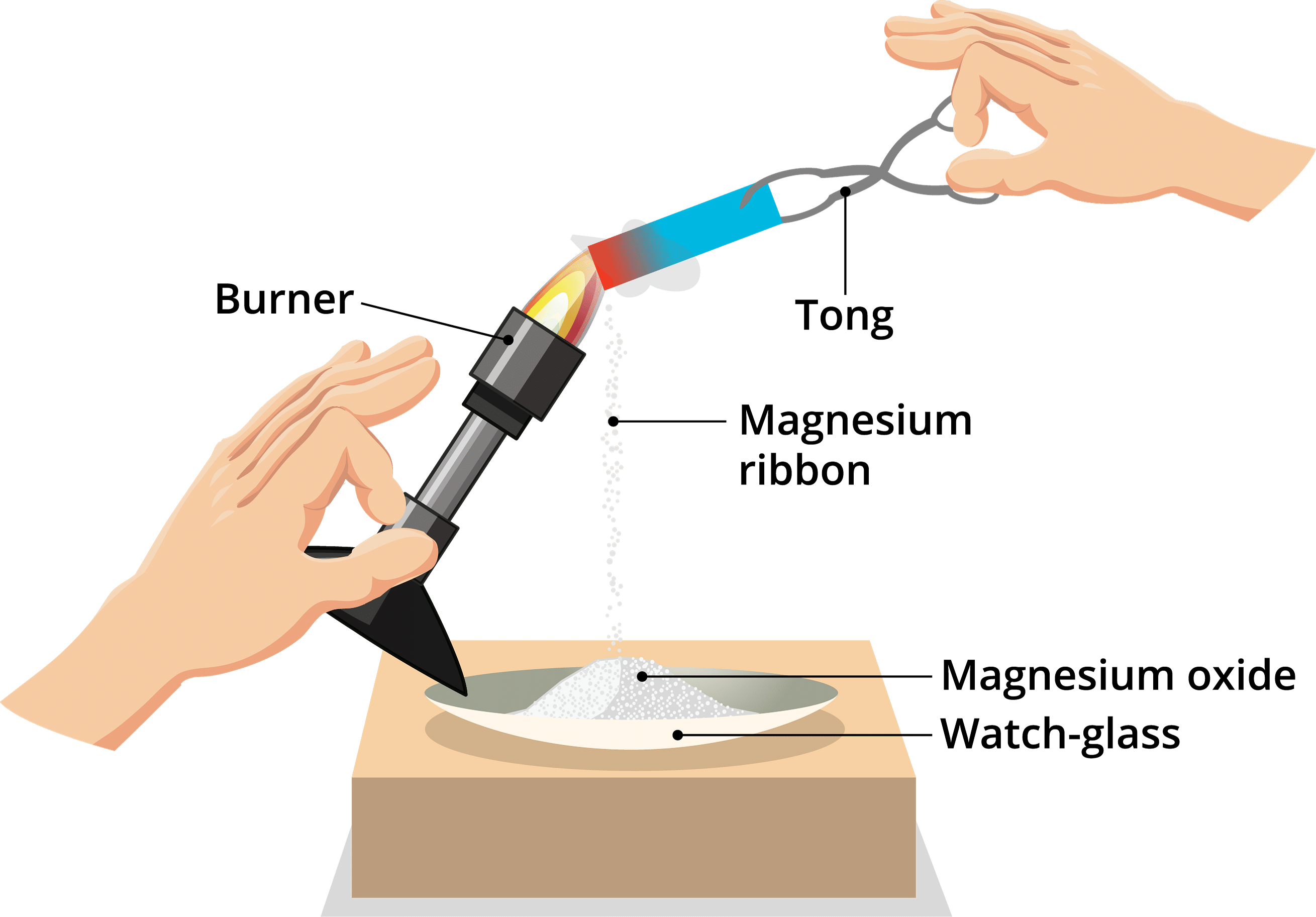
Here, X is magnesium oxide, and Y is magnesium nitride.
(a ) The chemical formulae of X are MgO and Y is Mg3N2.
(b ) When X is dissolved in water following reaction occurs.
MgO + H2O → Mg(OH)2
Q7: Zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not. Explain why?
Ans: Zinc is more reactive than copper as Zinc is placed above hydrogen, and copper is placed below hydrogen in the activity series of metals. Thus, zinc liberates hydrogen gas when reacted with dilute hydrochloric acid, whereas copper does not.
Q8: A silver article generally turns black when kept in the open for a few days. The article, when rubbed with toothpaste again, starts shining.
(a ) Why do silver articles turn black when kept in the open for a few days? Name the phenomenon involved.
(b ) Name the black substance formed and give its chemical formula.
Ans: (a ) The silver article turns black when kept in the air because the silver article reacts with sulphur compounds such as hydrogen sulphide (H2S) present in the air to form silver sulphide Ag2S. This phenomenon is called corrosion. It is also known as tarnishing of silver.
(b ) The black substance is silver sulphide. Its chemical formula is Ag2S.
Long Question Answer
Q1: When zinc granules are treated with a dilute solution of H2SO4, HCI, HNO3, NaCI and NaOH. Write the chemical equations if a reaction occurs.
Ans:

- Zinc granules react with dilute sulphuric acid to form zinc sulphate and hydrogen gas.
Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g) - Zinc granules react with dilute hydrochloric acid to form zinc chloride and hydrogen gas.
Zn (s) + H2SO4 (aq) → ZnCl2 (aq) + H2 (g) - Zinc granules react with dilute nitric acid to form zinc nitrate, water and dinitrogen gas.
Zn (s) + H2SO4 (aq) → Zn(NO3)2 (aq) + H2O (l) + N2O (g) - Zinc does not react with sodium chloride
Zn (s) + NaCl → No Reaction. - Zinc granules react with dilute sodium hydroxide to form zinc hydroxide and hydrogen gas.
Zn (s) + NaOH (aq) → Zn(OH)2 (aq) + Na (g)
Q2: What happens when a piece of
(a) Zinc metal is added to copper sulphate solution?
(b) Aluminium metal is added to dilute hydrochloric acid?
(c) Silver metal is added to copper sulphate solution?
Also, write the balanced chemical equation if the reaction occurs
Ans:
(a ) Zinc metal reacts with copper sulphate solution and forms colourless zinc sulphate and reddish-brown copper metal.
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
 (b ) Aluminium metal reacts with dilute hydrochloric acid to form aluminium chloride and hydrogen gas.2 Al (s) + 6 HCl (aq) → 2 AlCl3 (aq) + 3 H2 (g)
(b ) Aluminium metal reacts with dilute hydrochloric acid to form aluminium chloride and hydrogen gas.2 Al (s) + 6 HCl (aq) → 2 AlCl3 (aq) + 3 H2 (g)
(c ) Silver is less reactive than copper. Hence, no reaction will occur.
Q3: Give the characteristic tests for the following gases
(a ) CO2
(b ) SO2
(c ) O2
(d ) H2
Ans:
The characteristics test for
(a ) CO2: CO2 turns lime water milky due to the formation of insoluble calcium carbonate.
CO2 + Ca(OH)2 → CaCO3 + H2O

(b ) SO2: SO2 turns purple coloured acidic potassium permanganate solution colourless.
5 SO2 + 2 KMnO4 + 2 H2O → K2SO4 + 2 MnSO4 + 2 H2SO4
(c ) O2: We can confirm the evolution of oxygen gas by bringing a burning candle near the mouth of the test tube containing the reaction mixture. The intensity of the flame increases because oxygen supports burning.
(d ) H2: Hydrogen (H2) gas burns with a pop sound when a burning candle is brought near it.
Q4: On heating blue coloured powder of copper (I) nitrate in a boiling tube, copper oxide (black), oxygen gas, and a brown gas X is formed
(a) Write a balanced chemical equation of the reaction.
(b) Identity the brown gas X evolved.
(c) Identify the type of reaction.
(d) What could be the pH range of the aqueous solution of the gas X?
Ans:
(a) 2 CuNO3 (s) + Heat → 2 CuO (s) + 4 NO2 (g) + O2 (g)
 (b) The brown gas is of nitrogen dioxide.(c) It is a thermal decomposition reaction.(d) NO2 gas reacts with water to produce nitric acid. Thus, its pH range will be less than 7.
(b) The brown gas is of nitrogen dioxide.(c) It is a thermal decomposition reaction.(d) NO2 gas reacts with water to produce nitric acid. Thus, its pH range will be less than 7.
Q5: Balance the following chemical equations and identify the type of chemical reaction.
(a ) Mg (s) + Cl2 (g) → MgCI2 (s)
(b ) HgO (s) + Heat → Hg (l) + O2 (g)
(c ) Na (s) + S (s) → Na2S (s)
(d ) TlCl4 (l) + Mg (s) → Tl (s) + MgCl2 (s)
(e ) CaO (s) + SiO2 (s) → CaSiO3 (s)
(f ) H2O2 (l) + UV → H2O (l) + O2 (g)
Ans:
(a ) Mg (s) + Cl2 (g) → MgCI2 (s)
It is a combination reaction.
(b ) 2 HgO (s) + Heat → 2 Hg (l) + O2 (g)
It is a thermal decomposition reaction.
(c ) 2 Na (s) + S (s) → Na2S (s)
It is a combination reaction.
(d ) TlCl4 (l) + 2 Mg (s) → Tl (s) + 2 MgCl2 (s)
It is a displacement reaction.
(e ) CaO (s) + SiO2 (s) → CaSiO3 (s)
It is a combination reaction.
(f ) 2 H2O2 (l) + UV → 2 H2O (l) + O2 (g)
It is a decomposition reaction.
Q6: We made the following observations during the reaction of some metals with dilute hydrochloric acid.
(a) Silver metal does not show any change
(b) The temperature of the reaction mixture rises when aluminium (Al) is added.
(c) The sodium metal reaction is highly explosive.
(d) Some gas bubbles are seen when lead (Pb) is reacted with the acid.
Explain these observations giving suitable reasons.
Ans: (a) Silver does not show any characteristics change because silver is less reactive than hydrogen. Thus, it cannot displace hydrogen from dilute hydrochloric acid.
(b) The reaction between aluminium (Al) and hydrochloric acid is highly exothermic. Thus, the temperature of the reaction mixture rises.
(c) Sodium is a highly reactive metal. It reacts with hydrochloric acid, vigorously forming hydrogen gas and a large amount of heat.
(d) When lead reacts with hydrochloric acid, the gas bubbles observed are hydrogen gas.
Pb (s) + 2 HCl (aq) → PbCl2 (s) + H2 (g)
Q7: A white precipitate is obtained when adding a drop of barium chloride solution to an aqueous sodium sulphite solution.
(a ) Write a balanced chemical equation of the reaction involved
(b ) What other name can be given to this precipitation reaction?
(c ) On adding dilute hydrochloric acid to the reaction mixture, white residue disappears. Why?
Ans:
(a ) BaCl2 + Na2SO3 ⟶ BaSO3 + 2 NaCl
(b ) It can be assigned as a double displacement reaction.
(c ) On adding dilute hydrochloric acid to the reaction mixture, white residue disappears due to the formation of barium chloride.
BaSO3 + 2 HCl ⟶ BaCl2 + SO2 + H2O
|
85 videos|437 docs|75 tests
|
FAQs on Class 10 Science Chapter 1 Question Answers - Chemical Reactions and Equations
| 1. What is a chemical reaction? |  |
| 2. What are the different types of chemical reactions? |  |
| 3. How can chemical reactions be represented? |  |
| 4. What is the importance of balancing chemical equations? |  |
| 5. How can we identify a chemical reaction? |  |

|
Explore Courses for Class 10 exam
|

|













