Class 8 Science Chapter 11 Practice Question Answers - Chemical Effects of Electric Current
Q1: What are good conductors?
Ans: The substances that conduct electricity through them are called good conductors.
Q2: What are insulators or poor conductors?
Ans: The substances that do not conduct electricity through them are poor conductors or insulators.
Q3: Give four examples of conductors.
Ans: Copper, iron, aluminium and brass.
Q4: Give four examples of insulators.
Ans: Air, wood, rubber and plastic.
Q5: Name two metal objects which have a coating of another metal.
Ans: Handlebars of bicycles, bathroom taps.
Q6: What do we get on electrolysis of acidified water?
Ans: Hydrogen and oxygen gas.
Q7: Is air a bad or good conductor?
Ans: A bad conductor.
Q8: Which metal is plated on handle bars of cycles and rim of wheels?
Ans: Chromium
Q9: Substances that conduct electricity are called ___________.
Ans: conductors
Q10: Substances that do not conduct electricity are called ___________.
Ans: insulator
Q11: A cation has ___________ charge.
Ans: positive
Q12: Some liquids are ___________ conductors of electricity and some are ___________ conductors of electricity.
Ans: good, poor
Q13: Distilled water is an ___________.
Ans: insulator
Q14: Which of the following is a bad conductor of electricity?
(a) Distilled water
(b) Silver nitrate
(c) Sulphuric acid
(d) Copper sulphate
Ans: (a)
Explanation: Distilled water is a poor conductor of electricity. This is because it contains very few ions or charged particles that can carry an electric current. Pure water has a very low concentration of ions, making it a non-conductor or insulator.
Q15: Which of the following does not conduct electricity?
(a) Sugar solution
(b) Vinegar solution
(c) Lemon juice solution
(d) Caustic soda solution
Ans: (a)
Explanation: Sugar solution does not conduct electricity. Like distilled water, sugar solution does not have sufficient ions to allow the flow of electric current. Substances that do not conduct electricity are called insulators.
Q16: An electric current can produce
(a) heating effect
(b) chemical effect
(c) magnetic effect
(d) all of these
Ans: (d)
Explanation: An electric current can produce multiple effects: a heating effect (as seen in electric stoves or heaters), a chemical effect (electrolysis, where chemical reactions occur at the electrodes), and a magnetic effect (which results in the creation of a magnetic field around a current-carrying wire).
Q17: Pure or distilled water is a
(a) poor conductor
(b) good conductor
(c) both (a) and (b)
(d) none of these
Ans: (a)
Explanation: Pure or distilled water is a poor conductor of electricity due to its low ion concentration. While it can become conductive if impurities are dissolved in it, the pure form of water is considered a non-conductor.
Q18: Which of the following is a good conductor?
(a) Brick
(b) Steel
(c) Plastic
(d) Cotton
Ans: (b)
Explanation: Steel is a good conductor of electricity. Metals, in general, are good conductors because they have mobile electrons that can move freely within the material, allowing the flow of electric current. Steel is a metal and, therefore, a good conductor.
Value-Based Questions
Q19: Yakub made an circuit as shown in the figure. He observed that the bulb did not glow but on bringing a compass needle near it shows deflection.
He was quite confused that if current is flowing through the circuit then why the bulb is not glowing. Meanwhile his friend Sourav arrived and suggested him to add one more cell in the circuit. The bulb, then started glowing.
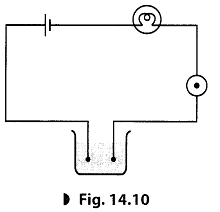
- Define a circuit.
- What does the deflection of a compass needle shows?
- Why the bulb did not glow in the first case but glow in the second case?
- What value of Sourav is shown here?
Ans:
- Circuit is a closed path through which an electric current flows.
- Deflection of compass needle shows that the current is flowing in the circuit. It is magnetic effect of current.
- The current flowing through the circuit in first case was too low to make the bulb glow but on adding a cell in the second case makes the bulb glow.
- Sourav is an intelligent, helpful, analytical and with scientific aptitude.
Q20: While demonstrating an experiment to show whether the given liquid conduct electricity or not to class VIII students, teacher reminded everybody that one should not conduct experiment with the electric supply from the mains or a generator or an inverter. They should use electric cells for the activity.
- Do liquids conduct electricity?
- Why we should not use electric source from mains generator or an inverter?
- What values do we get from this?
Ans:
- Yes, liquids which are solutions of acids, bases and salts conduct electricity. Other liquids such as oil, alcohol, sugar solution and pure water do not conduct electricity.
- Current flowing from mains, generator or an inverter is very large. So to avoid the chances of elec-trocution and short-circuit we must use cells for experiments.
- We get awareness of not using main electric supply and precaution to be followed while doing experiment.

|
Explore Courses for Class 8 exam
|

|

















