Class 5 EVS Chapter 7 HOTS Questions - Experiments with Water
Q1: What will happen chalk powder is dissolved in water?
(a) The chalk powder completely spread throughout the water
(b) Some chalk powder got settled down on the bottom and some got spread throughout the water
(c) The chalk powder gets completely settled down
(d) None of the above
Ans: (b) Some chalk powder settles at the bottom, and some spreads throughout the water.
 View Answer
View AnswerWhen chalk powder is added to water, it does not completely dissolve due to its larger particle size.
Observation:
- Initially, some chalk particles disperse in the water, creating a cloudy appearance.
- Over time, most of the chalk powder settles at the bottom while some remains suspended.
 Chalk Powder & Water
Chalk Powder & Water
Q2: ________ and water got mixed together to make the water look whitish.
(a) Turmeric
(b) Milk
(c) Black pepper
(d) None of the above
Ans: (b) Milk
 View Answer
View Answer- When milk is added to water, it mixes completely due to the polar nature of both substances.
- The milk contains fat globules that disperse in water, creating an opaque, whitish solution.
Q3: When water is added to the glass containing soil. What will happen?
(a) Initially, soil settles at the bottom and then dissolved in water
(b) Soil settles down at the bottom of the glass
(c) Soil completely dissolved in water
(d) None of the above
Ans: (b) Soil settles down at the bottom of the glass
 View Answer
View Answer- Soil particles are larger and denser than water, so they do not dissolve.
- When water is added, soil will initially float on the surface but will eventually settle at the bottom.
 Soil Water Mixture
Soil Water Mixture
Q4: Puffed puri will float on the water as it is lighter than water.
(a) True
(b) False
Ans: (a) True
 View Answer
View Answer- Puffed puri is lighter than water, which allows it to float.
- The air trapped inside the puri reduces its overall density, enabling it to stay on the water's surface.
Q5: Identify the substance(s) that dissolved by water.
(a) Salt
(b) Honey
(c) Sugar
(d) All of these
Ans: (d) All of these
 View Answer
View Answer- Water is often called the "universal solvent" because it can dissolve many substances.
- Examples of substances that dissolve in water include:
- Salt: Dissolves easily, making the water salty.
- Sugar: Dissolves completely, making the water sweet.
- Honey: Also mixes well in water.
- Therefore, the answer is (d) All of these.
Q6: Brianna was trying to dissolve salt and water. What will she observe?
(a) Salt and water got mixed together completely
(b) Some salt settles at the bottom, and rest mixed with water
(c) Salt settles at the bottom
(d) None of the above
Ans: (a) salt and water got mixed together completely
 View Answer
View Answer- When Brianna adds salt to water, she will see that the salt completely dissolves.
- This means that the salt will mix with the water and become part of it, creating a salty solution.
- So, the correct observation is (a) salt and water got mixed together completely.
 Salt and Water
Salt and Water
Q7: The empty plastic bottle will float on the water. What will happen with the bottle filled with water?
(a) It will also float on the water
(b) It will sink in the water
(c) It will first float on the water, and then sink in the water
(d) None of the above
Ans: (b) It will sink in the water
 View Answer
View Answer- An empty plastic bottle floats because it is lightweight.
- However, when the bottle is filled with water, it becomes heavier.
- Because it weighs more than the water, the filled bottle will sink. Thus, the answer is (b) It will sink in the water.

Q8: Oil floats as a layer on the surface of the water.
(a) True
(b) False
Ans: (a) True
 View Answer
View Answer- This statement is True. Oil floats on top of water because it is less dense than water.
- When oil is poured into water, it forms a layer on the surface instead of mixing in, making it easy to see.
 Oil & Water
Oil & Water
Q9: Suggest some ways to Hamid for quickly dissolving of sugar.
Ans: To help Hamid dissolve sugar more quickly, he can follow these methods:
Stir the Mixture Thoroughly:
- Stirring increases the contact between sugar and water, allowing the sugar molecules to disperse more effectively.
- Use a spoon or a stirrer to mix the solution continuously.
Warm the Mixture:
- Heating the water increases the kinetic energy of the molecules, which helps dissolve the sugar faster.
- Care should be taken not to boil the water, as it may cause loss of water through evaporation.
Use Smaller Sugar Particles:
- If using granulated sugar, Hamid can crush or use powdered sugar, which has a larger surface area and dissolves more quickly.
Q10: Would the cap of plastic bottle cap sink or float on water?
Ans:
- The cap will float on water.
- The cap is lightweight compared to the water, which allows it to float.
- The buoyant force acting on the cap is greater than its weight, causing it to remain on the surface.
Q11: An object made of glass is immersed in water. Will it float or sink?
Ans:
- When a piece of glass is placed in water, it sinks because it is heavier than water. This is because glass has a higher density than water, meaning it has more mass in the same amount of space.
- However, when glass is put in mercury, it floats. This is because mercury is heavier than glass. Therefore, glass has a lower density than mercury.
Q12: What things do you put in water to make tea? Which of those things dissolves in water?
Ans: To make a cup of tea, you typically need:
- Tea leaves: For flavor.
- Sugar: To sweeten the tea.
- Milk: To make it creamy.
- Among these ingredients, sugar and milk dissolve in water:
- Sugar dissolves completely, making the tea sweet.
- Milk mixes well, giving the tea a creamy texture.
- The tea leaves don’t dissolve but add flavor to the water.
Q13: Do you think the oil got dissolved in the water? Why do you think so?
Ans:
- No, the oil does not dissolve in water.
- When you pour oil into water, it floats on top instead of mixing in.
- This happens because oil is less dense than water, which means it does not mix and stays as a separate layer on the surface.
Q14: Make a group of four friends. For the experiment, you will need 4-5 glasses or bowls and the things listed in the table. Take some water in each glass. Now try to dissolve one thing in one glass. Observe that happens and note in the table.
Ans:
Objective: To observe the solubility of different substances in water.
Materials Needed:
- 4-5 glasses or bowls
- Various substances (e.g., salt, sugar, sand, oil, chalk powder)
Steps:
- Gather a group of four friends.
- Fill each glass with an equal amount of water.
- Add a different substance to each glass.
- Stir each mixture gently and observe what happens.
- Note the observations in a table format.
Sample Observations: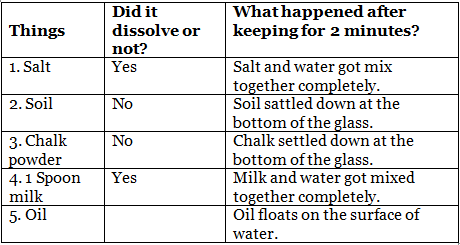
|
35 videos|240 docs|41 tests
|
FAQs on Class 5 EVS Chapter 7 HOTS Questions - Experiments with Water
| 1. What are the different states of water observed in experiments? |  |
| 2. How can we demonstrate that water expands when it freezes? |  |
| 3. What happens when we heat water, and how can we observe this in an experiment? |  |
| 4. Why does ice float on water, and how can we explain this phenomenon? |  |
| 5. What are some common experiments we can do with water at home? |  |

|
Explore Courses for Class 5 exam
|

|

















