Textbook Solutions: Matter | Eureka Plus Class 5: Book Solutions, Notes & Worksheets PDF Download
I. Tick (✔) the correct options.
1. Molecules of any substance are made of __________
(a) big particles
(b) atoms
(c) ball and sticks
(d) elements
Ans: (b)
2. A molecule having two or more types of atoms belongs to __________
(a) iron metal
(b) a compound
(c) oxygen gas
(d) an element
Ans: (b)
3. The molecules of hydrogen have only one type of atom because hydrogen is a / an __________
(a) gas
(b) compound
(c) element
(d) matter
Ans: (c)
4. The molecules are closely arranged in ________
(a) oils
(b) gases
(c) solids
(d) elements
Ans: (c)
5. When heated slightly, it melts. It could be _________
(a) paper
(b) an iron nail
(c) bee wax
(d) a pebble
Ans: (c)
II. Tick (✔) the true sentences and correct the false ones.
1. Only the things that we can see are made up of matter.
Ans: False
Anything we can see, touch, or smell is matter.
2. Every element has a symbol.
Ans: True
3. In a solid, the molecules are arranged far away from each other.
Ans: False
In a solid, the molecules are tightly packed.
4. In a gas, the force of attraction between the molecules is very weak.
Ans: True
5. A chemical change forms a new substance.
Ans: True
6. The symbol of chlorine is Ch.
Ans: False
The symbol of chlorine is Cl.
III. Answer the following questions in one sentence.
1. Name the molecule that has two hydrogen atoms and one oxygen atom.
Ans: Water molecule.
2. Write the symbols of the elements that form a carbon dioxide molecule.
Ans: C (carbon) and O (oxygen).
3. Which state of matter does not have any definite shape but has a definite volume?
Ans: Liquid.
4. What are the three states of water?
Ans: Solid (ice), liquid, and gas (water vapor).
5. What is a physical change?
Ans: A physical change is a temporary change that does not result in the formation of a new substance, and the properties of the substance do not change.
IV. Answer the following questions.
1. How does an element differ from a compound?
Ans: An element is a substance whose molecules are made of only one kind of atom, while a compound is a substance whose molecules are made of more than one kind of atom.
2. What determines the state of matter?
Ans: The arrangement of molecules and the force of attraction between them determine the state of matter.
3. Why do liquids flow?
Ans: Liquids flow because the force of attraction between the molecules is weak enough to allow them to move slightly away from each other, allowing the liquid to flow.
4. A solid substance changes to a liquid on heating. Why?
Ans: Heating provides energy to the molecules, causing them to move further apart, changing the solid into a liquid.
5. What kind of change is mostly a temporary change? Explain such a change with an example.
Ans: A physical change is mostly a temporary change. For example, melting wax is a physical change, as the wax can solidify again upon cooling.
V. Do as directed.
1. Draw a diagram to describe the molecule of oxygen and justify why oxygen is an element. Name another element.
Ans: 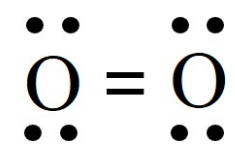
Oxygen is an element because its molecules are made of only one kind of atom, which is oxygen (O).
Another element: Hydrogen (H).
2. Draw a diagram of the ammonia molecule and justify why ammonia is a compound. Give another example of a compound.
Ans: 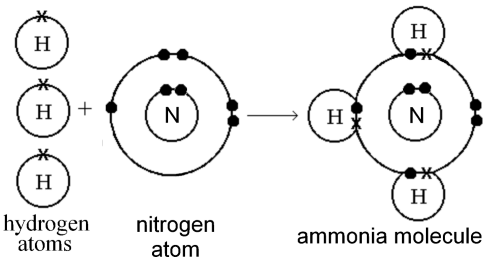
Ammonia is a compound because its molecules are made of more than one kind of atom (nitrogen and hydrogen).
Another example of a compound: Water (H2O).
3. Explain why the molecules of carbon dioxide gas spread throughout the entire available space.
Ans: The molecules of carbon dioxide gas spread because there is hardly any force of attraction between the molecules, allowing them to move freely and occupy the available space.
4. Give an example of a chemical change. Explain why it is a chemical change.
Ans: Heating sugar until it turns into a black substance (carbon). It is a chemical change because a new substance (carbon) with different properties is formed, and the process is irreversible.)
Think and answer
I. Four sets of illustrations are given below. Each shows the arrangement of molecules in a certain space of the same size (A, B, C, D). Different atoms are coloured differently. Study the illustrations and answer the questions that follow.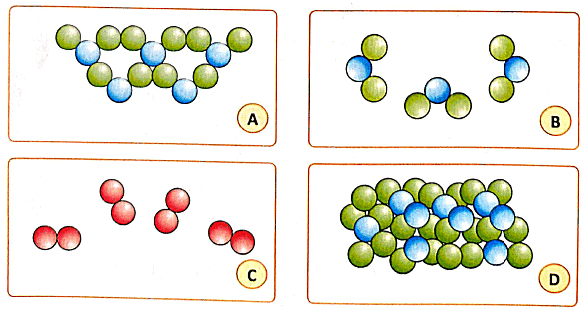
1 . Which box represents an element?
(a) [A]
(b) [B]
(c) [C]
(c) [D]
Ans: (b)
An element is a substance whose molecules are made up of only one kind of atom. In the given illustrations, Box B represents an element because it shows molecules composed of two green atoms, suggesting they are molecules of the same element, possibly oxygen or nitrogen based on common diatomic elements.
2. What could be the state of this element?
(a) Solid
(b) Liquid
(c) Gas
Ans: (c)
The state of matter (solid, liquid, or gas) is determined by the arrangement and force of attraction between the molecules. Since the molecules in Box B are relatively far apart compared to those in Box A, it could be a gas. This is supported by the fact that gases like oxygen and nitrogen are diatomic and exist as gases at room temperature.
3. Which three sets represent the same substance?
(a) [A]
(b) [B]
(c) [C]
(c) [D]
Ans: (a, b, d)
The same substance would be represented by the same molecular composition and arrangement. Boxes A, B, and D represent the same substance since they all contain green and blue beads, which could represent the same types of atoms in different states (solid, gas, and liquid respectively).
4. Which set do you think represents a solid substance?
(a) [A]
(b) [B]
(c) [C]
(c) [D]
Ans: (a)
A solid substance has molecules that are closely packed due to strong forces of attraction between them. Box A represents a solid substance because the molecules are tightly packed together.
5. Which set could be a liquid
(a) [A]
(b) [B]
(c) [C]
(c) [D]
Ans: (d)
A liquid has a weaker force of attraction between molecules than a solid, allowing some movement and resulting in no definite shape but a definite volume. Box D could represent a liquid because the molecules are not as tightly packed as in a solid, allowing for some movement between them.
II. In your notebook, draw the correct figure if the given one is incorrect.
1. The ball and stick models of molecules of an element
Ans: If the illustration shows molecules made up of only one kind of atom (same colored balls), then it would be correct for an element. However, if it shows molecules made up of different kinds of atoms (different colored balls), it would be incorrect for an element.
2.
The ball and stick models of molecules of a compound.
Ans: A correct illustration would show molecules made up of more than one kind of atom (different colored balls connected together). If the illustration shows molecules made up of only one kind of atom, it would be incorrect for a compound.
3. The ball and stick models of molecules of two different elements.
Ans: This should show two different kinds of molecules, each made of the same kind of atoms but different from each other (for example, two red balls representing oxygen molecules and two white balls representing hydrogen molecules). If the illustration shows the same kind of molecule or molecules with different atoms connected together, it would be incorrect.
4.
The ball and stick models of molecules of two different compounds
Ans: A correct illustration for this would show two sets of molecules, each made up of two or more different kinds of atoms (for example, one set with one black and two red balls representing carbon dioxide, and another set with two white and one red ball representing water). If the illustration shows molecules made up of only one kind of atom or does not show two different compounds, it would be incorrect.





















