Shankar IAS Summary: Ozone Depletion- 2 - BPSC (Bihar) PDF Download
| Table of contents |

|
| Introduction |

|
| Why Antarctic Ozone Depletion Occurs? |

|
| Arctic Ozone Depletion |

|
| The Ozone Depleting Substances Rules |

|
Introduction
Ozone is a unique form of oxygen with three atoms bound together. It is present in two layers of the atmosphere. In the troposphere, it's considered "bad" because it contributes to air pollution and smog. In the stratosphere, it's "good" as it protects life by absorbing harmful UV rays from the sun.
 Ozone layer depletion in atmosphere
Ozone layer depletion in atmosphere
The ozone layer is crucial because it acts like a sunscreen, efficiently absorbing UV light. UV rays can damage DNA, harm the immune system, and lead to diseases. Ozone prevents UV radiation from reaching Earth's surface, reducing the risk of mutations and harm to plants and animals.
Measuring ozone involves various instruments like the Dobson spectrophotometer, M83 filter ozonometer, and TOMS on the Nimbus-7 satellite. The Dobson unit is a common measure, representing the thickness of the ozone layer in milli-centimeters. It helps gauge ozone abundance, with one Dobson unit equal to 2.69 x 1020 molecules per square meter at Standard Temperature and Pressure.
Change in Equilibrium
- The equilibrium between ozone formation and destruction is disrupted by certain substances entering the atmosphere.
- These substances react with ozone, causing its destruction.
- The rate of ozone destruction exceeds its natural formation rate.
- This imbalance results in a significant decrease in ozone concentration, known as 'Ozone Depletion.'
- Over Antarctica, for example, the atmosphere now contains only about 50 percent of its original ozone.
- The awareness of ozone depletion became clear in 1985.
Chlorofluorocarbons (CFCs)
 CFC molecule
CFC molecule
- Chlorofluorocarbons (CFCs) are molecules made up of chlorine, fluorine, and carbon.
- Uses: CFCs are utilized as refrigerants, aerosol propellents, foaming agents in plastic manufacturing, fire extinguishers, solvents for cleaning electronics and metals, and in freezing foods.
- CFC Distribution: About two-thirds of CFCs are used as refrigerants, and one-third are used as blowing agents in foam insulation products.
- Why Used: CFCs are chosen for various applications due to their properties like non-corrosiveness, non-inflammability, low toxicity, and chemical stability.
- Lifetime & Removal: Unlike other chemicals, CFCs cannot be easily removed from the atmosphere through typical processes. Their estimated residence time in the atmosphere is between 40 and 150 years. They move from the troposphere to the stratosphere during this time.
- Escape into the Atmosphere: CFCs gradually evaporate from sources, such as discarded refrigerators. They can survive in the troposphere, but exposure to UV radiation in the stratosphere leads to their breakdown.
- Chemical Reaction: When exposed to UV radiation, CFC molecules break up, releasing chlorine atoms. These chlorine atoms react with ozone molecules, forming chlorine monoxide and eventually leading to the depletion of ozone.

- Depletion Process: Ozone depletion is catalytic, with chlorine being reformed at the end of the cycle. A single chlorine atom can destroy thousands of ozone molecules before being neutralized by reactive nitrogen or hydrogen compounds.
- CFC Substitutes: Alternatives to CFCs should be safe, cost-effective, energy-efficient, and have low ozone layer depletion potential (ODP) and low global warming potential (GWP). HFC 134a (R-134a) is a promising substitute for CFC-12 (R-12), along with alternatives like (R-143a) and (R-152a).
Nitrogen Oxides (NOx)
- Sources: Mainly from thermonuclear explosions, industrial emissions, and agricultural fertilizers.
- Chemical Reaction: Nitric oxide (NO) catalytically destroys ozone through a reaction with ozone and oxygen.

- Escape of NO: Nitrous oxide (N2O) is released from solid sources through denitrification and nitrification processes, reaching the stratosphere where it is photolytically destroyed to yield nitric oxide, further contributing to ozone destruction.
Other Substances
- Bromine Compounds:
- Halons, HBFCs (hydrobromo fluorocarbons), and methyl bromide (a pesticide) release bromine atoms that destroy ozone molecules more effectively than chlorine atoms.
- Bromine combines with ozone, forming bromine monoxide (BrO) and oxygen (O2), which further reacts with chlorine monoxide (ClO), releasing free atoms of bromine and chlorine that can react with ozone.
- Sulphuric Acid Particles: These particles free chlorine and convert reactive nitrogen into inert forms, preventing the formation of chlorine reservoirs.
- Other Substances: Carbon tetrachloride and methyl chloroform, both toxic solvents, and aerosols contribute to ozone depletion.
 Carbon Tetrachloride molecule
Carbon Tetrachloride molecule
Monitoring the Ozone Layer: Various organizations contribute to monitoring the atmosphere and exchanging information about the ozone layer, including:
- World Weather Watch (WWW)
- Integrated Global Ocean Services Systems (IGOSS)
- Global Climate Observing System (GCOS)
- World Meteorological Organization (WMO)
Why Antarctic Ozone Depletion Occurs?
 Antarctic ozone hole over the years
Antarctic ozone hole over the years
- Colder Antarctic Stratosphere:
- The Antarctic stratosphere is much colder than other regions.
- Low temperatures enable the formation of Polar Stratospheric Clouds (PSCs) below 20 km.
- Ozone Absorption of Sunlight:
- Ozone absorbs sunlight, causing an increase in temperature with altitude in the stratosphere.
- If ozone is depleted, the air becomes cooler, creating conditions favorable for PSC formation and stabilizing the vortex.
- Vortex Formation and Longevity:
- The Antarctic vortex, a ring of rapidly circulating air, confines ozone depletion in the Antarctic region.
- The longevity of the Antarctic vortex is crucial, lasting throughout the polar winter into mid-spring, while the Arctic vortex disintegrates by polar spring (March-April).
- Winter Months in Antarctica Leading to Ozone Depletion:
- June: Antarctic winter begins, vortex develops, and temperature falls, allowing clouds to form.
- July and August: PSCs denitrify and dehydrate the stratosphere, and hydrochloric acid and chlorine nitrate react on cloud surfaces to free chlorine.
- September: Sunlight returns, PSCs disappear due to rising temperatures. ClO-ClO and ClO-BrO catalytic cycles destroy ozone. The lowest ozone levels are reached in October.
- November: Polar vortex breaks down, ozone-rich air from mid-latitudes replenishes the Antarctic stratosphere, and ozone-poor air spreads over the southern hemisphere.
Arctic Ozone Depletion
 Ozone hole over Arctic region
Ozone hole over Arctic region
- Increasing Evidence:
- Ozone depletion is increasingly observed in the Arctic.
- In March 1996, the Arctic experienced the greatest ozone depletion ever seen in the northern hemisphere.
- Causes:
- Scientists attribute past Arctic ozone depletion to a significant cooling of the upper atmosphere in northern latitudes.
- Ozone depletion over the northern hemisphere has been steadily increasing since the winter of 1992.
- Main Contributing Factors:
- Besides the accumulation of ozone-depleting chemicals, a crucial factor is the rising cold temperature in the Arctic stratosphere.
- Cold temperatures encourage the formation of Polar Stratospheric Clouds (PSCs), contributing to ozone depletion.
A decrease in the quantity of total-column ozone tends to cause increased penetration of solar UV-B radiation (290-315nm) to the earth’s surface. UV-B radiation is the most energetic component of sunlight reaching the earth’s surface. It has profound effects on human health, animals, plants, micro-organisms, materials, and on air quality.
Effects on Human and Animal Health
Potential Risks:
- Increased incidence and morbidity from eye diseases, skin cancer, and infectious diseases.
- UV radiation damages the cornea and lens of the eye.
- UV exposure decreases immune response to skin cancers and infectious agents, leading to unresponsiveness upon repeated challenges.
- Light-skinned populations are susceptible to non-melanoma skin cancer due to UV-B radiation.
 Adverse effects of ozone depletion on human health
Adverse effects of ozone depletion on human health
Effects on Terrestrial Plants
(i) Psychological and Developmental Processes:
- UV-B radiation affects the psychological and developmental processes in plants.
- Variability in Response:
- UV-B response varies among plant species and cultivars.
- Agriculture may require UV-B tolerant cultivars and breeding new ones.
(ii) Impact on Ecosystems:
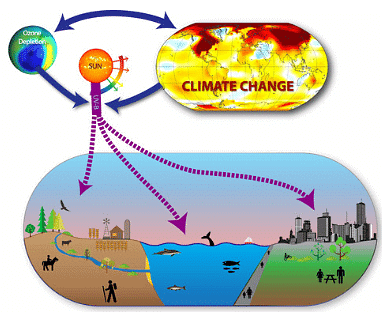 Ozone depletion impact on ecosystems
Ozone depletion impact on ecosystems
- Changes in species composition in forests and grasslands may occur, affecting biodiversity.
- Indirect changes in plant form, biomass allocation, timing of developmental phases, and second metabolism are important.
Effects on Aquatic Ecosystems
Phytoplankton Orientation and Motility:
- Solar UV-B radiation affects orientation and motility in phytoplankton, reducing their survival rates.
- Early developmental stages of fish, shrimp, crab, amphibians, and other animals are damaged, resulting in decreased reproductive capacity and impaired larval development.
Effects on Bio-Geochemical Cycles
Alteration of Cycles:
- Increased solar UV radiation could impact terrestrial and aquatic biogeochemical cycles, altering sources and sinks of greenhouse and chemically important trace gases.
- Potential changes may contribute to biosphere-atmosphere feedback, reinforcing atmospheric build-up of these gases.
Effects on Air Quality
Tropospheric Changes:
- Reduction in stratospheric ozone and increased UV-B radiation penetrating to the lower atmosphere affect photodissociation rates of key trace gases.
- This can increase the production and destruction of ozone and related oxidants, with adverse effects on human health, plants, and materials.
- Changes in atmospheric concentrations of the hydroxyl radical may affect atmospheric lifetimes of climatically important gases.
- Increased tropospheric reactivity could lead to increased production of particulates, affecting air quality.
Effects on Materials
Adverse Impact on Materials:
- Synthetic polymers, bio-polymers, and other commercial materials are adversely affected by solar UV radiation.
- Routine exposure to sunlight demands the use of light stabilizers and/or surface treatment to protect materials, such as plastics.
- Any increase in solar UV-B content due to partial ozone depletion accelerates photodegradation rates, limiting the outdoor life of these materials.
The Ozone Depleting Substances Rules
The Ozone Depleting Substances (Regulation and Control) Rules, 2000, were established under the Environment (Protection) Act in July 2000.
- Objectives:
- These rules set deadlines for phasing out various Ozone Depleting Substances (ODSs) and regulate their production, trade, import, export, and usage in products.
- Amendments in 2001, 2003, 2004, and 2005 aimed to facilitate the implementation of ODS phase-out across different sectors.
- Prohibitions and Deadlines:
- Prohibit the use of CFCs in manufacturing various products beyond January 1, 2003, except for metered dose inhalers and medical purposes.
- Prohibit the use of halons after January 1, 2001, except for essential purposes. Other ODSs like carbon tetrachloride and methylchloroform, along with CFCs for metered dose inhalers, allowed until January 1, 2010.
- Methyl bromide use allowed until January 1, 2015. HCFCs, interim substitutes for CFCs, permitted until January 1, 2040.
- Ozone Depleting Substances Amendment Rules, 2019: Under the Environment (Protection) Act of 1986, the Ozone Depleting Substances (Regulation and Control) Amendment Rules, 2019, have been published.
- Import License Prohibition: According to the Amendment Rules, the Ministry of Environment, Forest, and Climate Change (MoEFCC) informed that the issuance of import licenses for HCFC-141b is prohibited from January 1, 2020.
- India's Achievements:
- India successfully phased out Hydrochlorofluorocarbon (HCFC)-141b, a chemical used by foam-producing businesses, by January 1, 2020.
- India consciously chose to move away from ozone-depleting substances, opting for eco-friendly and energy-efficient technologies.
FAQs on Shankar IAS Summary: Ozone Depletion- 2 - BPSC (Bihar)
| 1. Why does Antarctic ozone depletion occur? |  |
| 2. What are the causes of Arctic ozone depletion? |  |
| 3. What are the Ozone Depleting Substances (ODS) rules? |  |
| 4. How does ozone depletion occur? |  |
| 5. What are some effects of ozone depletion? |  |





















