2021
Q1: A sample (5.6 g) containing iron is completely dissolved in cold dilute HCl to prepare a 250 mL of solution. Titration of 25.0 mL of this solution requires 12.5 mL of 0.03 M KMnO4 solution to reach the end point. Number of moles of Fe2+ present in 250 mL solution is x 10-2 (consider complete dissolution of FeCl2). The amount of iron present in the sample is y% by weight.
(Assume: KMnO4 reacts only with Fe2+ in the solution
Use : Molar mass of iron as 56 g mol-1) [JEE Advanced 2021 Paper 2]
The value of x is ______.
Ans: 1.875
Using relation,
n2M1V1 = n1M2V2 [n1 and n2 are number of moles of Fe2+ and MnO-4 reacting]
The volume of x is 1.88.
Q2: A sample (5.6 g) containing iron is completely dissolved in cold dilute HCl to prepare a 250 mL of solution. Titration of 25.0 mL of this solution requires 12.5 mL of 0.03 M KMnO4 solution to reach the end point. Number of moles of Fe2+ present in 250 mL solution is x 10-2 (consider complete dissolution of FeCl2). The amount of iron present in the sample is y% by weight.
(Assume : KMnO4 reacts only with Fe2+ in the solution
Use : Molar mass of iron as 56 g mol-1)
The value of y is ______. [JEE Advanced 2021 Paper 2]
Ans: 18.75
Mass of sample = 5.6 g
Number of moles of Fe = 1.88 x 10-2 mol
Molar mass of Fe = 56 g/mol
Mass of Fe = Moles of Fe x Molar mass

⇒ y = 18.75%
The value of y is 18.75.
Q3: Ozonolysis of ClO2 produces an oxide of chlorine. The average oxidation state of chlorine in this oxide is __________. [JEE Advanced 2021 Paper 2]
Ans: 6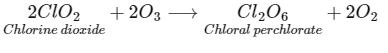
Oxidation state of Cl in Cl2O6 = 2x + 6(2) = 0
2x - 12 = 0
2x = 12 ⇒ x = + 6
Average oxidation state of Cl in Cl2O6 is 6.
2020
Q1: Choose the correct statement(s) among the following: [JEE Advanced 2020 Paper 2]
(a) SnCl2 . 2H2O is a reducing agent.
(b) SnO2 reacts with KOH to form K2[Sn(OH)6].
(c) A solution of PbCl2 in HCl contains Pb2+ and Cl− ions.
(d) The reaction of Pb3O4 with hot dilute nitric acid to give PbO2 is a redox reaction.
Ans: (a, b)
Sn2+ of stannous chloride dihydrate (SnCl2 . 2H2O) tends to convert into Sn4+.
Hence, statement (a) is correct.
(b) SnO2 reacts with KOH and gives K2SnO3 . 3H2O or K2[Sn(OH)6] because it is amphoteric in nature.

Hence, statement (b) is correct.
(c) In conc. HCl, PbCl2 exists as chloroplumbous acid, H2[PbCl4]
Hence, statement (c) is incorrect.
(d) Pb3O4 is a mixture of 

It is not a redox reaction. Thus, the statement (d) is incorrect.

|
Explore Courses for JEE exam
|

|
















