Calorimeter | Chemistry for EmSAT Achieve PDF Download
Calorimetry is the scientific discipline concerned with gauging a body's thermal characteristics to assess its physical and chemical alterations, which may range from physical transitions like melting and evaporation to chemical reactions such as combustion or acid-base neutralization. A calorimeter serves as the instrument for quantifying these thermal changes. This branch of science finds wide application in thermochemistry for determining parameters like enthalpy, stability, and heat capacity.
What Is a Calorimeter?
A calorimeter serves as a tool for conducting heat measurements essential in calorimetry. Typically, it comprises a metallic container crafted from materials with high electrical conductivity, like copper or aluminum. Additionally, it features a stirring mechanism for homogenizing the contents. The metallic vessel, along with the stirrer, is encased within an insulating jacket to minimize heat dissipation to the surroundings. A single aperture allows for the insertion of a thermometer to monitor alterations in thermal characteristics internally. Now, let's delve into the process of conducting heat measurements. In the preceding article, we explored the specific heat capacity of various substances.
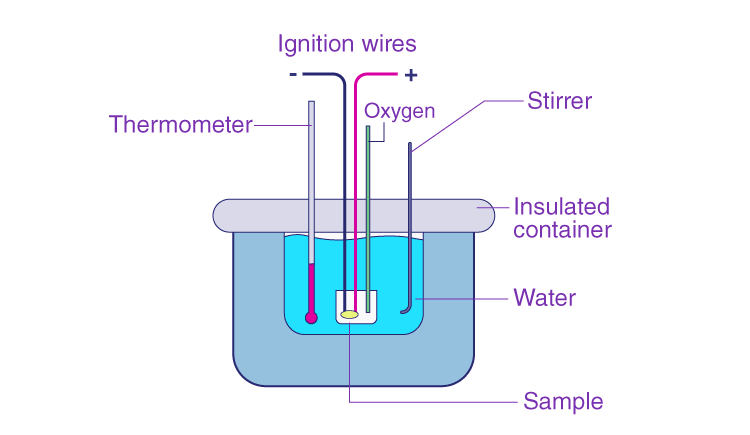
These measurements can be conducted effortlessly using this method. For instance, within a calorimeter, a predetermined quantity of fuel undergoes combustion. The vessel containing water is heated as a result of this combustion process. The principle of heat conservation dictates that the heat lost by the fuel equals the heat gained by the water. Hence, insulating the calorimeter from the surroundings is crucial to enhance experimental precision. The alteration in heat can be quantified using a thermometer. Through such measurements, we can determine both the heat capacity of water and the energy content stored within the fuel.
Uses of Calorimetry
It is widely acknowledged that matter invariably adheres to the principle of attaining the lowest possible energy state. Nevertheless, matter exhibits a spectrum of energetic states, as exemplified by uranium atoms, which possess considerable energy potential.
The energy content of matter significantly influences its natural prevalence and reactivity, among other factors. By deciphering the interplay between energy and these properties, we can forecast the natural prevalence, reactivity, and physical attributes based on energy assessments conducted via calorimetry. A comprehensive comprehension of a substance's thermodynamic characteristics inevitably provides insights into its structure and other properties.
Types of Calorimeter
Various categories of calorimeters include:
- Adiabatic Calorimeters
- Reaction Calorimeters
- Bomb Calorimeters (also known as Constant Volume Calorimeters)
- Constant Pressure Calorimeters
- Differential Scanning Calorimeter
|
191 videos|265 docs|160 tests
|















