Grade 11 Exam > Grade 11 Notes > Chemistry for Grade 11 (IGCSE) > Preparation of Insoluble Salts
Preparation of Insoluble Salts | Chemistry for Grade 11 (IGCSE) PDF Download
Preparing Insoluble Salts
- Insoluble salts are formed using a precipitation reaction.
- The solid salt obtained is known as the precipitate. To ensure success, the solid salt formed must be insoluble in water, and the reactants must be soluble.
When two soluble reactants are used, a precipitation reaction occurs. The diagram below illustrates the filtration process to separate the precipitate:
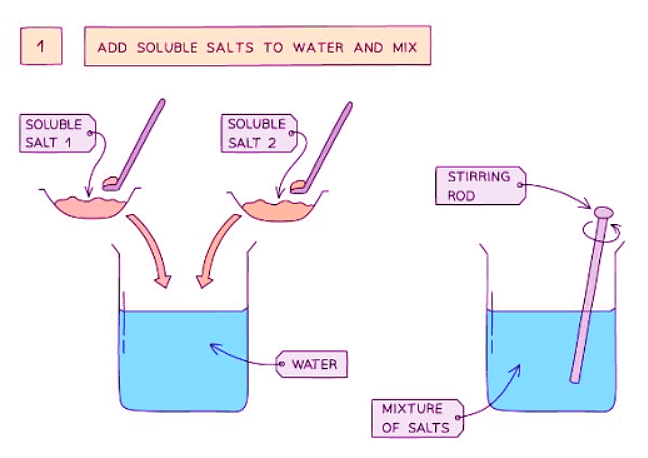



Method
Follow these steps to prepare insoluble salts:
- Mix two soluble reactants in a solution.
- A precipitation reaction will occur, forming an insoluble salt as a solid.
- Filter the mixture to separate the precipitate from the solution.
- Collect and dry the precipitate to obtain the insoluble salt.
Question for Preparation of Insoluble SaltsTry yourself: What is the purpose of the filtration process in preparing insoluble salts?View Solution
Example: Preparation of pure, dry lead(II) sulfate crystals using a precipitation reaction
Soluble Salts Used
- Soluble Salt 1: lead(II) nitrate
- Soluble Salt 2: potassium sulfate
Method
- Dissolve lead(II) nitrate and potassium sulfate in water. Mix them well in a beaker using a stirring rod.
- Filter the mixture to separate the precipitate.
- Wash the precipitate with distilled water to remove any traces of potassium nitrate solution.
- Allow the washed precipitate to dry in an oven.
Equation of reaction
lead(II) nitrate + potassium sulfate → lead(II) sulfate + potassium nitrate
Pb(NO3)2(aq) + K2SO4(aq) → PbSO4(s) + 2KNO3(aq)
The document Preparation of Insoluble Salts | Chemistry for Grade 11 (IGCSE) is a part of the Grade 11 Course Chemistry for Grade 11 (IGCSE).
All you need of Grade 11 at this link: Grade 11
|
103 docs|53 tests
|
FAQs on Preparation of Insoluble Salts - Chemistry for Grade 11 (IGCSE)
| 1. What are insoluble salts? |  |
Ans. Insoluble salts are salts that do not dissolve in water or any other solvent to a significant extent.
| 2. How can insoluble salts be prepared? |  |
Ans. Insoluble salts can be prepared by mixing two solutions of soluble salts that react to form an insoluble salt, which can then be precipitated out.
| 3. Can insoluble salts be separated from a solution? |  |
Ans. Yes, insoluble salts can be separated from a solution through a process called precipitation, where the insoluble salt forms a solid that can be filtered out.
| 4. What are some common examples of insoluble salts? |  |
Ans. Some common examples of insoluble salts include silver chloride (AgCl), lead(II) sulfate (PbSO4), and calcium carbonate (CaCO3).
| 5. Why are insoluble salts useful in chemistry? |  |
Ans. Insoluble salts are useful in chemistry for various purposes, such as forming precipitates for qualitative analysis, as catalysts, or as pigments in paints and dyes.

|
Explore Courses for Grade 11 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches
















