Year 7 Exam > Year 7 Notes > Developing the Periodic Table
Developing the Periodic Table - Year 7 PDF Download
Introduction
- All elements are pure substances made from only one type of atom and are listed on the periodic table. They are arranged in a chart called the periodic table based on their structure and properties.
- The arrangement of elements in the periodic table is determined by their atomic structure and unique properties.
- In the 19th century, Dmitri Mendeleev, a Russian scientist, developed one of the earliest practical periodic tables, organizing elements in a systematic way.
The development of the periodic table
- In the 1800s, scientists discovered numerous new elements, which are pure substances composed of only one type of atom and are organized on the periodic table. However, at that time, there was no systematic way to categorize these elements. Scientists endeavored to identify similarities in their properties to arrange them meaningfully.
Early attempts to organize the elements
- John Newlands observed that when elements were arranged by atomic weight (a measure of an atom's mass), every eighth element exhibited similar characteristics. For instance, sodium, positioned eight places after lithium, displayed comparable reactions.
- An illustrative example of this pattern is seen in the reactions of lithium (Li), sodium (Na), and potassium (K) with water, where all three elements exhibit similar behavior.

Julius Meyer's Contribution
- Julius Meyer organized elements based on their atomic weight and the number of atoms, which are the smallest particles of an element. Atoms are not just tiny spheres; they consist of even smaller particles like protons, neutrons, and electrons.
- Some elements are grouped together because they tend to combine with two other atoms. For instance, one oxygen atom can bond with two hydrogen atoms to form water (H2O).

Question for Developing the Periodic TableTry yourself: Which scientist organized elements based on their atomic weight and the number of atoms they consist of?View Solution
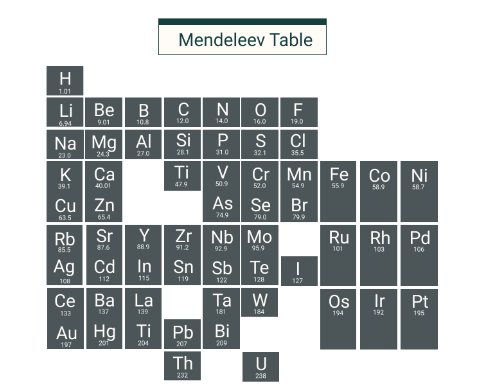
Mendeleev's Periodic Table

A Russian scientist named Dmitri Mendeleev developed one of the earliest practical periodic tables in the 19th century. The contemporary periodic table is closely based on his concepts.
- The elements are organized in ascending order of atomic mass.
- The horizontal rows are termed periods.
- The vertical columns are referred to as groups.
- Elements within the same group exhibit similarities.
Mendeleev's principles remain relevant today. However, the modern periodic table organizes elements by increasing atomic number rather than atomic mass.

How Mendeleev Developed the Periodic Table
- Mendeleev arranged element names on cards to identify patterns. He ordered the cards from lightest to heaviest element and grouped them based on each element's chemical and physical properties.
- Chemical properties refer to how an element or compound reacts with other substances, while physical properties are observable or measurable characteristics such as melting point, electrical conductivity, and appearance at room temperature.
FAQs on Developing the Periodic Table - Year 7
| 1. How was the periodic table developed? |  |
Ans. The periodic table was developed through the collaboration of multiple scientists over time, with Dmitri Mendeleev creating the first widely-recognized version in 1869.
| 2. What is the relationship between chemical elements and atoms in the periodic table? |  |
Ans. Chemical elements are substances that consist of only one type of atom, and the periodic table organizes these elements based on the number of protons in their atoms.
| 3. What role do the chemical and physical properties of elements play in the periodic table? |  |
Ans. The periodic table groups elements with similar chemical and physical properties together, making it easier to predict the behavior of elements based on their position in the table.
| 4. Who is credited with creating the modern periodic table as we know it today? |  |
Ans. Dmitri Mendeleev is credited with creating the modern periodic table, arranging elements by atomic mass and leaving gaps for undiscovered elements that were later confirmed.
| 5. What interesting fact about the periodic table is often overlooked? |  |
Ans. One interesting fact is that the periodic table is not just a list of elements, but a powerful tool that helps scientists understand the fundamental building blocks of matter and predict their properties.
Download as PDF

|
Explore Courses for Year 7 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches
















