Enzyme and its Classification | Biology Class 11 - NEET PDF Download
| Table of contents |

|
| What are Enzymes? |

|
| Chemical Reactions |

|
| Nature of Enzyme Action |

|
| Factors affecting Enzyme Activity |

|
| Classification of Enzymes |

|
| Co- factors |

|
What are Enzymes?
Enzymes are biological molecules that act as catalysts, which speed up chemical reactions in living organisms by lowering the activation energy needed, thus increasing the rate of reactions without being consumed themselves.
Structure
- Enymes are made up of polypeptide chains, which are long chains of amino acids connected by peptide bonds.
- These proteins have complex three-dimensional structures formed by the folding and coiling of amino acid chains.
- A small part of the enzyme's structure, known as the active site, is involved in catalysis.
- This site includes both the catalytic and binding sites where substrates bind and reactions are facilitated.
Chemical Reactions
1. Enzymes are like helpers in chemical reactions. When certain substances mix, they can change into new substances. This change involves breaking old bonds and forming new ones. For example, when you mix a chemical called Ba(OH)2 with another called H2SO4, you get BaSO4 and water. That's a chemical reaction.
2. There are also physical changes, like when ice melts into water. These don't involve breaking bonds, just changing shape or state. The speed at which these changes happen is called the rate. Temperature affects how fast these changes occur - generally, a 10°C change doubles or halves the rate.
3. Now, back to enzymes. They speed up chemical reactions. Without them, some reactions would be super slow. For instance, without an enzyme called carbonic anhydrase, it takes ages for carbon dioxide and water to become carbonic acid. But with this enzyme, it happens super fast - millions of times faster!
4. Enzymes are amazing because there are thousands of them, each doing a specific job. Sometimes, several enzymes work together in what's called a metabolic pathway. This is like a series of steps, with each step catalyzed by a different enzyme. For example, glucose turning into pyruvic acid involves ten steps, each with its enzyme.
5. These pathways can lead to different end products depending on conditions. For instance, in muscles without enough oxygen, lactic acid forms. But in normal conditions, it's pyruvic acid. And in yeast during fermentation, it's ethanol. So, the same pathway can lead to different results depending on the situation.
How do Enzymes bring about such High Rates of Chemical Conversions?
- Enzymes are like tiny machines in our bodies that help speed up chemical reactions. Imagine them as workers on an assembly line, where the starting material (substrate) needs to be transformed into a product. The substrate binds to a specific site on the enzyme, creating a temporary "ES" complex. This binding helps the substrate change its shape into a transition state, where the actual chemical changes happen.
- When we plot this on a graph, with energy on the y-axis and the different stages of the transformation on the x-axis, we can see that the energy level of the starting material (S) is higher than that of the end product (P) in some cases.
- If P is lower than S, it's like rolling downhill, releasing energy as heat.
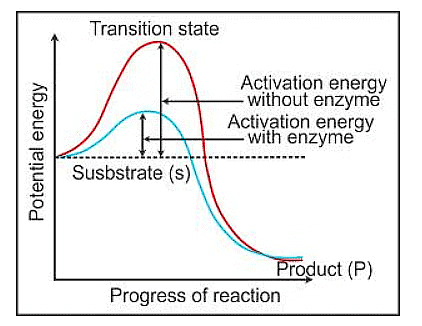
- But regardless of whether the reaction releases or absorbs energy, the substrate still has to go through a high-energy stage called the transition state. Enzymes help by lowering this energy barrier, making it easier for the substrate to turn into the product.
Nature of Enzyme Action
Each enzyme possesses a specific site where its substrate binds, leading to the formation of a transient enzyme-substrate complex. This complex quickly breaks down into products and the original enzyme, with an intermediate enzyme-product complex formed during the process. The formation of this enzyme-substrate complex is crucial for catalyzing the reaction.
The enzyme's catalytic cycle involves several steps:
- Initially, the substrate attaches to the enzyme's active site, fitting snugly into place.
- The binding of the substrate prompts the enzyme to undergo a conformational change, tightening its grip around the substrate.
- With the substrate held closely, the enzyme catalyzes the breaking of chemical bonds, resulting in the formation of an enzyme-product complex.
- The enzyme then releases the reaction products, returning to its original state and becoming available to bind with another substrate molecule, restarting the catalytic cycle.
Factors affecting Enzyme Activity
(a) Temperature and pH Effects
Enzyme activity is highly susceptible to alterations in temperature and pH levels. These changes can disrupt the protein's tertiary structure, affecting its functionality. Enzymes typically operate optimally within specific temperature and pH ranges, with activity diminishing outside these parameters. Extreme temperatures can either temporarily inhibit (at low temperatures) or permanently denature (at high temperatures) enzyme function.
(b) Impact of Substrate Concentration
The rate of enzymatic reactions initially increases with rising substrate concentration until it reaches a maximum velocity (Vmax). Beyond this point, further substrate increases do not augment reaction velocity. This plateau occurs because enzyme molecules become saturated with substrate molecules, leaving no available enzyme sites for additional substrates to bind.
(c) Chemical Influences: Inhibition
Enzyme activity can also be modulated by specific chemicals that bind to the enzyme, resulting in inhibition. Inhibition occurs when a chemical hampers enzyme function. Competitive inhibition involves inhibitors that closely resemble the substrate in molecular structure. These inhibitors compete with the substrate for binding sites on the enzyme, hindering substrate binding and subsequently reducing enzyme activity. For instance, malonate, which resembles succinate, competitively inhibits succinic dehydrogenase, thereby impeding the enzyme's action. Competitive inhibitors are commonly utilized in controlling bacterial pathogens.
Classification of Enzymes
Enzymes are categorized into six major classes based on the type of reaction they catalyze:
1. Oxidoreductases: Catalyze oxidation-reduction reactions where electrons are transferred between molecules.
2. Transferases: Catalyze the transfer of functional groups between molecules.
3. Hydrolases: Catalyze the hydrolysis of chemical bonds.
4. Lyases: Catalyze reactions that form or break double bonds.
5. Isomerases: Catalyze the rearrangement of atoms within a molecule.
6. Ligases: Catalyze the joining of two large molecules by forming a new chemical bond.
Co- factors
Enzymes consist of one or multiple polypeptide chains, but in certain cases, non-protein components known as cofactors are necessary for the enzyme to function catalytically. When these cofactors are bound to the enzyme, the protein part is referred to as the apoenzyme. There are three types of cofactors: prosthetic groups, coenzymes, and metal ions.
- Prosthetic groups are organic compounds tightly bound to the apoenzyme. For instance, in enzymes like peroxidase and catalase, which break down hydrogen peroxide, the prosthetic group haem is an integral part of the enzyme's active site.
- Coenzymes are also organic compounds, but they only transiently associate with the apoenzyme, usually during catalysis. They participate in various enzyme-catalyzed reactions, with many containing essential vitamins, such as nicotinamide adenine dinucleotide (NAD) and NADP, which include niacin.
- Certain enzymes rely on metal ions for their activity, forming coordination bonds with side chains at the active site and sometimes with the substrate itself. For example, zinc acts as a cofactor for the proteolytic enzyme carboxypeptidase.
- The removal of a cofactor from an enzyme results in the loss of catalytic activity, highlighting the crucial role these cofactors play in enzyme function.
|
183 videos|524 docs|136 tests
|
FAQs on Enzyme and its Classification - Biology Class 11 - NEET
| 1. What are enzymes and how do they work in chemical reactions? |  |
| 2. How does the nature of enzyme action contribute to their role in biochemical processes? |  |
| 3. What factors can affect enzyme activity and how do they influence the efficiency of enzyme-catalyzed reactions? |  |
| 4. How are enzymes classified based on their functions and structure, and what are some examples of different enzyme classes? |  |
| 5. What are co-factors and how do they play a role in enzyme activity and regulation? |  |















