Year 7 Exam > Year 7 Notes > Year 7 Chemistry (Cambridge) > Neutralisation reactions
Neutralisation reactions | Year 7 Chemistry (Cambridge) PDF Download
| Table of contents |

|
| Introduction to pH Scale and Universal Indicator |

|
| Experiment: Demonstrating Neutralization |

|
| Specific Neutralization Reactions |

|
| Naming Salts in Neutralization Reactions |

|
Introduction to pH Scale and Universal Indicator
pH Scale Overview:
- Acids range from pH 0 to 6, indicated by colors red (strong acid) to yellow (weak acid).
- Neutral substances like pure water are at pH 7.
- Alkalis range from pH 8 to 14, shown in colors from dark green to blue and purple.
Experiment: Demonstrating Neutralization
Setup and Procedure:
- Two solutions used: an acid (red with universal indicator) and an alkali (green/blue with universal indicator).
- Adding alkali to acid gradually to achieve neutralization.
- Observing color changes: red to green indicating neutralization, purple for excess alkali, and weak acid colors for excess acid.
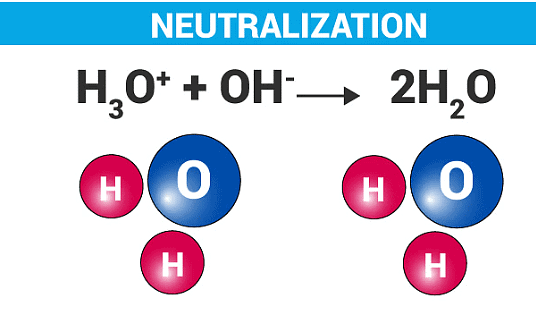
Everyday Applications of Neutralization
Example: Indigestion Tablets:
- Dissolve in saliva to form alkali.
- Neutralize excess stomach acid upon ingestion.
Chemistry Behind Neutralization
- General Reaction Formula:
- Acid + Alkali → Salt + Water.
- Understanding Terms:
- Base that dissolves in water is an alkali.
- Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
Question for Neutralisation reactionsTry yourself: Which of the following substances is an example of an alkali?View Solution
Specific Neutralization Reactions
- Hydrochloric Acid (HCl):
- Hydrochloric acid + Sodium hydroxide (NaOH) → Sodium chloride (NaCl) + Water.
- Hydrochloric acid + Calcium hydroxide (Ca(OH)2) → Calcium chloride (CaCl2) + Water.
- Hydrochloric acid + Potassium hydroxide (KOH) → Potassium chloride (KCl) + Water.
- Sulfuric Acid (H2SO4):
- Sulfuric acid + Sodium hydroxide (NaOH) → Sodium sulfate (Na2SO4) + Water.
- Sulfuric acid + Calcium hydroxide (Ca(OH)2) → Calcium sulfate (CaSO4) + Water.
- Sulfuric acid + Potassium hydroxide (KOH) → Potassium sulfate (K2SO4) + Water.
- Nitric Acid (HNO₃):
- Nitric acid + Sodium hydroxide (NaOH) → Sodium nitrate (NaNO3) + Water.
- Nitric acid + Calcium hydroxide (Ca(OH)2) → Calcium nitrate (Ca(NO3)2) + Water.
- Nitric acid + Potassium hydroxide (KOH) → Potassium nitrate (KNO3) + Water.
Naming Salts in Neutralization Reactions
Rule:
- Hydrochloric acid makes salts ending in chloride.
- Sulfuric acid makes salts ending in sulfate.
- Nitric acid makes salts ending in nitrate.
The document Neutralisation reactions | Year 7 Chemistry (Cambridge) is a part of the Year 7 Course Year 7 Chemistry (Cambridge).
All you need of Year 7 at this link: Year 7
|
7 videos|7 docs|7 tests
|
FAQs on Neutralisation reactions - Year 7 Chemistry (Cambridge)
| 1. What is a neutralization reaction? |  |
Ans. A neutralization reaction is a chemical reaction between an acid and a base that results in the formation of water and a salt. This reaction effectively neutralizes the acidic and basic properties of the initial substances.
| 2. How can you identify a neutralization reaction? |  |
Ans. A neutralization reaction can be identified by the formation of water and a salt as products. Additionally, the pH of the solution will shift towards being neutral (pH 7) after the reaction takes place.
| 3. What are some common examples of neutralization reactions in everyday life? |  |
Ans. Some common examples of neutralization reactions include the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) to form water and sodium chloride (NaCl), which is table salt. Another example is the reaction between acetic acid (vinegar) and sodium bicarbonate (baking soda) to produce water, carbon dioxide, and sodium acetate.
| 4. How does the concentration of acids and bases affect a neutralization reaction? |  |
Ans. The concentration of acids and bases can impact the rate and extent of a neutralization reaction. Higher concentrations of acids and bases can lead to faster reaction rates and the production of more salts and water.
| 5. Can you explain the role of indicators in neutralization reactions? |  |
Ans. Indicators are substances that change color in response to the pH of a solution. In neutralization reactions, indicators can be used to visually determine when the reaction has reached completion, typically by changing color from acidic to basic or vice versa.

|
Explore Courses for Year 7 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches
















