NCERT Solutions for Class 9 Science Chapter 4 - Structure of the Atom
| Table of contents |

|
| Page No. 39 |

|
| Page No. 41 |

|
| Page No. 42 |

|
| Page No. 44 |

|
| Page No. 45 |

|
| Page No. 46, 47 & 48 |

|
Page No. 39
Q1. What are canal rays?
Ans: Canal rays are a type of positively charged radiation discovered by E. Goldstein in 1886. They are produced in a gas discharge and are significant for the following reasons:
- They helped in the identification of protons, a key subatomic particle.
- Canal rays consist of positive ions that move towards the cathode in a discharge tube.
- Their discovery contributed to the understanding of atomic structure.
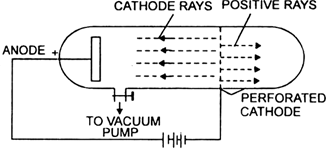 Goldstein's experimental setup
Goldstein's experimental setup
Q2. If an atom contains one electron and one proton, will it carry any charge or not?
Ans: No, it will not carry any charge because the number of protons (positively charged) is equal to the number of electrons (negatively charged).
Page No. 41
Q1. On the basis of Thomson's model of an atom, explain how the atom is neutral as a whole.
Ans: According to Thomson's model, an atom is made up of a sphere of positive charge with electrons embedded within it. This arrangement is designed to create a stable electrostatic balance.
- The atom is neutral because it contains an equal number of electrons and positively charged particles.
- The positive charge of the sphere balances the negative charge of the electrons.
- Thus, the overall charge of the atom is zero, making it electrically neutral.
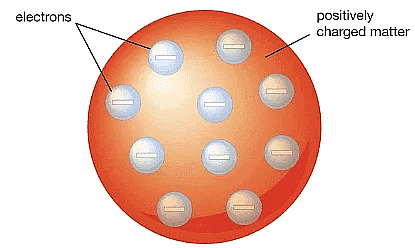 Thomson's Model of an atom
Thomson's Model of an atom
Q2. On the basis of Rutherford's model of an atom, which subatomic particle is present in the nucleus of an atom?
Ans: According to Rutherford's model of the atom:
- Protons are present in the nucleus, giving it a positive charge.
- The nucleus contains most of the atom's mass.
- Neutrons are also found in the nucleus, contributing to the overall mass.
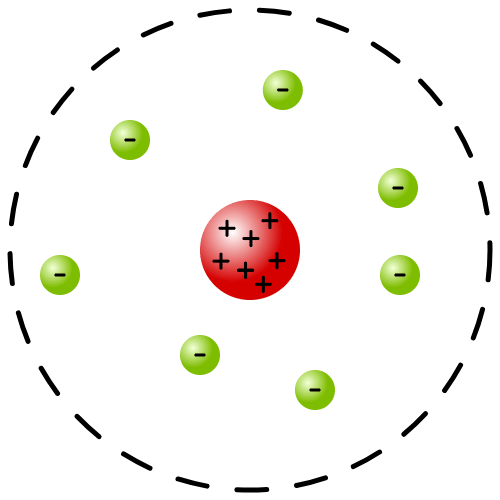 Rutherford's Model of an atom
Rutherford's Model of an atom
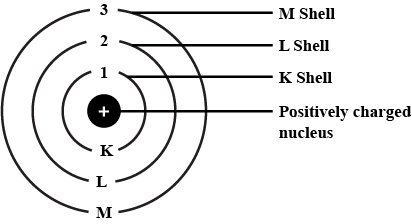
Q3. Draw a sketch of Bohr's model of an atom with three shells.
Ans:
Q4. What do you think would be the observation if the α-particle scattering experiment is carried out using a foil of a metal other than gold?
Ans: If the metal taken is heavier than gold, then the deviation shown by α-particles will be more, and if the metal taken is lighter than gold, then the deviation shown will be less due to less repulsion between positively α-particles and nucleus.
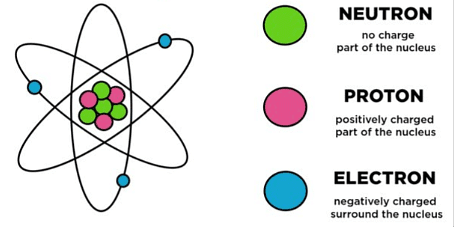
Q5. Name the three sub-atomic particles of an atom.
Ans: The three sub-atomic particles of an atom are:
(i) Protons
(ii) Electrons
(iii) Neutrons
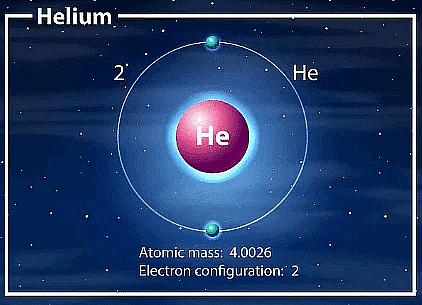
Q6. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Ans: Number of neutrons = Atomic mass - Number of protons
For a helium atom:
- Atomic mass = 4 u
- Number of protons = 2
- Therefore, number of neutrons = 4 - 2 = 2
Page No. 42
Q1. Write the distribution of electrons in carbon and sodium atoms.
Ans:
(a) Electronic distribution in a carbon atom:
- Atomic number: 6
- Number of electrons: 6
- Distribution: K = 2, L = 4
(b) Distribution of electrons in a sodium atom:
- Atomic number: 11
- Number of electrons: 11
- Distribution: K = 2, L = 8, M = 1
Q2. If K and L shell of an atom are full, then what would be the total number of electrons in the atom?
Ans. The maximum number of electrons in an atom with full K and L shells is:
- K shell: 2 electrons
- L shell: 8 electrons
Therefore, the total number of electrons in the atom is:
- Total: 10 electrons
Page No. 44
Q1. How will you find the valency of chlorine, sulphur and magnesium?
Ans: To find the valency of chlorine, sulphur, and magnesium:
Chlorine (Cl):
- Atomic number: 17
- Electrons: 2, 8, 7
- Can gain 1 electron to achieve stability (like argon).
- Valency: 1
Sulphur (S):
- Atomic number: 16
- Electrons: 2, 8, 6
- Can gain 2 electrons to achieve stability (like argon).
- Valency: 2
Magnesium (Mg):
- Atomic number: 12
- Electrons: 2, 8, 2
- Can lose 2 electrons to achieve stability (like neon).
- Valency: 2
Q2. If number of electrons in an atom is 8 and number of protons is also 8, then
(i) what is the atomic number of the atom and
(ii) what is the charge on the atom?
Ans:
(i) The atomic number is equal to the number of protons in an atom. In this case:
Atomic number = Number of protons = 8
(ii) The charge of an atom is determined by the balance between protons and electrons.
- Since the number of electrons (8) is equal to the number of protons (8), the atom has no overall charge.
- Thus, the charge on the atom is zero.
Q3. With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Ans:
(a) To find the mass number of Oxygen:
Number of protons = 8
Number of neutrons = 8
Atomic number = 8
Atomic mass number = Number of protons + number of neutrons = 8 + 8 = 16
Therefore, mass number of oxygen = 16
(b) To find the mass number of Sulphur:
Number of protons = 16
Number of neutrons = 16
Atomic number = 16
Atomic mass number = Number of protons + number of neutrons = 16 + 16 = 32
Page No. 45
Q1. For the symbol H, D and T tabulate three sub-atomic particles found in each of them.
Ans:
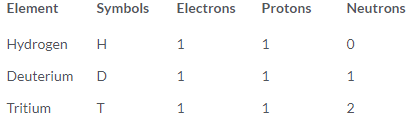
Hydrogen (H): The most common isotope, consisting of a single proton and no neutrons.
Deuterium (D): A heavier isotope of hydrogen, with one neutron in addition to the proton.
Tritium (T): A radioactive isotope of hydrogen, containing two neutrons and one proton.
Q2. Write the electronic configuration of any one pair of isotopes and isobars.
Ans:
Isotopes : Isotopes have the same electronic configuration because they have the same number of electrons and protons, differing only in the number of neutrons.
Example : 12C6 and 14C6 are isotopes, have the same electronic configuration as (2, 4)
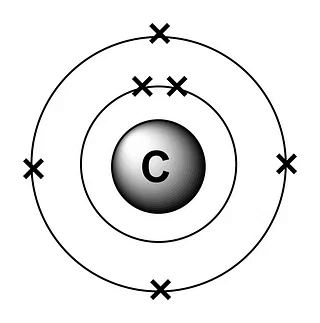 Electronic Configuration(2,4) of Carbon atom
Electronic Configuration(2,4) of Carbon atom
Isobars: Isobars have the same mass number but differ in atomic number, leading to different electronic configurations.
Example : 22Ne10 and 22Na11 are isobars. They have different atomic number but mass number is same.
Page No. 46, 47 & 48
Q1. Compare the properties of electrons, protons and neutrons.
Ans:
| Electron | Proton | Neutron |
| (a) It is negatively charged. | (a) It is positively charged. | (a) It is neutral. |
| (b) Its absolute mass is equal to 9.1 x 10-31 kg. | (b) Its absolute mass is equal to 1.673 x 10-27 kg. | (b) Its mass is slightly more than that of protons. Its absolute mass is 1.675 x 10-27 kg. |
Q2. What are the limitations of J.J. Thomson's model of the atom?
Ans: Limitations of JJ Thomson's model of an atom:
(i) The results of experiments carried out by other scientists could not be explained by this model
(ii) The positive charge is spread all over could not be justified.
Q3. What are the limitations of Rutherford's model of the atom?
Ans: Limitations of Rutherford's model of an atom
(i) According to classical electromagnetic theory, a moving charged particle, such as an electron, under the influence of attractive force, loses energy continuously in the form of radiations. As a result of this, the electron should lose energy and, therefore, should move in even smaller orbits, ultimately falling into the nucleus. But the collapse does not occur. There is no explanation for this behaviour.
(ii) According to Rutherford, the electrons revolve around the nucleus in fixed orbits. However, Rutherford did not specify the number of orbits and the number of electrons in each orbit.
Q4. Describe Bohr's model of the atom.
Ans: Bohr made a bold new suggestion that particles at the atomic level would behave differently from macroscopic (bigger) objects.
According to Bohr’s theory:
- Electrons travel in specific paths called orbits or energy levels.
- While in these orbits, electrons do not emit energy.
- Electrons can move to a higher energy level when they absorb energy.
- When electrons drop back to a lower energy level, they release energy.
This model helped explain the stability of atoms and their emission spectra.
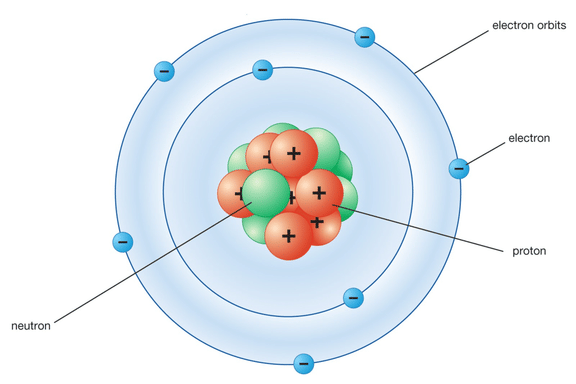 Bohr's Model of an atom
Bohr's Model of an atom
Q5. Compare all the proposed models of an atom given in this chapter.
Ans:
| Thomson model | Rutherford model | Bohr model |
| (a) The positive charge is spread all over and electrons are embedded in it like seeds in the watermelon. | (a) There is a positively charged centre called the nucleus where the mass of the atom is concentrated. | (a) Electrons revolve in certain discrete orbits called energy levels. |
| (b ) The positive and negative charges are equal, therefore the atom is neutral. | (b) Electrons revolve around the nucleus in well-defined orbits. | (b) While revolving in these orbits, electrons do not radiate energy. |
| (c) The nucleus is very small compared to the overall size of the atom. | (c) The size of the nucleus is very small as compared to the size of an atom. | (c) These orbits are represented by K, L, M, N or 1, 2, 3, 4. |
Q6. Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Ans: The distribution of electrons in the shells of an atom is known as electronic configuration. To write the electronic configuration for an element, follow these steps:
- Determine the total number of electrons using the element's atomic number.
- Distribute these electrons across the shells in order of increasing energy, following the 2n2 rule.
- Fill the shell with the lowest energy first, then proceed to the next higher energy shells.
- List the number of electrons in each shell, starting from the lowest, separated by commas. For example, sodium (atomic number 11) has:
Electronic configuration of Na: K = 2, L = 8, M = 1
The maximum number of electrons in different shells is:
- K-shell (1st shell) = 2
- L-shell (2nd shell) = 8
- M-shell (3rd shell) = 18
- N-shell (4th shell) = 32
Key points to remember:
- The outermost shell can hold a maximum of 8 electrons.
- Electrons fill shells in a step-wise manner; inner shells must be filled before outer shells.
Q7. Define valency by taking examples of silicon and oxygen.
Ans: The valency of an atom is the number of hydrogen atoms which combine with one atom of an element.
For example,
- Silicon (atomic number 14) has the following electronic distribution:
K = 2, L = 8, M = 4.
In the outermost shell, there are 4 electrons; hence, one atom of silicon will combine with 4 atoms of hydrogen. So, the valency of silicon is 4. In the case of oxygen (atomic number 8), the electronic distribution in various shells is given below:
K = 2, L = 6
There are six valence electrons in the atom of oxygen. So, one atom of oxygen will combine with 2 atoms of hydrogen to make a noble gas configuration. Hence, the valency of oxygen is 2.
Q8. Explain with examples (i) Atomic number, (ii) Mass number, (iii) Isotopes and (iv) Isobars. Give any two uses of isotopes.
Ans:
(i) Atomic number: The atomic number of an atom is equal to the number of protons in the nucleus of an atom.
Atomic number = Number of protons.
(ii) Mass number: The total mass of the atom is due to the sum of the masses of protons and neutrons present in the nucleus. The mass number of an atom is equal to the sum of the number of protons and neutrons in the nucleus.
Mass number = Number of protons + Number of neutrons.
(iii) Isotopes: Isotopes are the atoms of the same element having the same atomic number but different mass numbers. For example, Hydrogen has three isotopes: protium (1), deuterium (2), and tritium (3).
(iv) Isobars: Isobars are the atoms of the different elements having the same mass number and different atomic numbers. For example, Calcium (atomic number 20) and argon (atomic number 18) both have a mass number of 40.
Uses of isotopes:
(i) Cobalt-60 is a radioactive isotope of cobalt. It is used in radiotherapy for cancer.
(ii) 14C is a radioactive isotope of carbon. It is used in carbon dating.
Q9. Na+ has completely filled K and L shells. Explain.
Ans: Sodium has the atomic number 11. It has 11 electrons in its orbitals, wherein the number of protons is equal to the number of electrons. Hence, its electronic configuration is K-2, L-8, and M-1. The one electron in the M shell is lost, and it obtains a positive charge since it has one more proton than electrons and obtains a positive charge, Na+. Na+ is formed when one electron is lost by a sodium atom, as given below: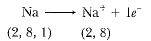
The new electronic configuration is K-1; L-8 which is the filled state. Hence it is very difficult to eliminate the electron from a filled state as it is very stable.
Q10. If bromine atom is available in the form of, say, two isotopes 79Br35 (49.7%) and 81Br35 (50.3%) calculate the average atomic mass of bromine atom.
Ans: Average atomic mass of Br
= ( (79 x 49.7) + (81 x 50.3) ) / 100
= (3926.3 + 4074.3) / 100
= 8000.6 / 100
= 80.006 u
Q11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 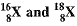 in the sample?
in the sample?
Ans: It is given that the average atomic mass of the sample of element X is 16.2 u.
Let the percentage of isotope 18 / 8 X be y%. Thus, the percentage of isotope 16 / 8 X will be (100 - y) %.
Therefore,
18 x (y/100) + 16 x ((100-y)/100) = 16.2
18y/100 + ( 16(100 - y) / 100 ) = 16.2
(18y+1600-16y )/100 = 16.2
18y + 1600 - 16y = 1620
2y + 1600 = 1620
2y = 1620 - 1600
y = 10
Therefore, the percentage of isotope 18 / 8 X is 10%.
And, the percentage of isotope 16 / 8 X is (100 - 10) % = 90%.
Q12. If Z = 3, what would be the valency of the element? Also, name the element.
Ans. Z = 3
Electronic configuration is 2, 1.
Valence electron is equal to 1.
Valency is also equal to 1.
The name of the element is lithium.
Q13. Composition of the nuclei of two atomic species X and Y are given as under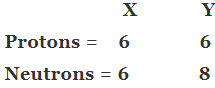
Give the mass numbers of X and Y. What is the relation between the two species?
Ans: Mass number of X = 6 + 6 = 12
Mass number of Y = 6 + 8 = 14
Atomic number of X = 6
Atomic number of Y = 6
X and Y are isotopes since both the elements have same atomic number but different mass number.
Q14. For the following statements, write T for 'True' and F for 'False'.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1 / 2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Ans. (a) False (b) False (c) True (d) False
Q15. Rutherford's alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic nucleus
(b) Electron
(c) Proton
(d) Neutron
Ans: (a) Atomic nucleus
An atomic nucleus is the dense central core of an atom, composed of protons and neutrons held together by the strong nuclear force.
Q16. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers
Ans: (c) different number of neutrons
Q17. Number of valence electrons in Cl -ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Ans: (b) 8
Cl- has 8 valence electrons. Cl- has 17 + 1 = 18 electrons. Its electronic configuration is 2, 8, 8.
Q18. Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1
Ans: (d) 2 ,8 ,1
2 ,8 ,1 is the correct electronic configuration of sodium.
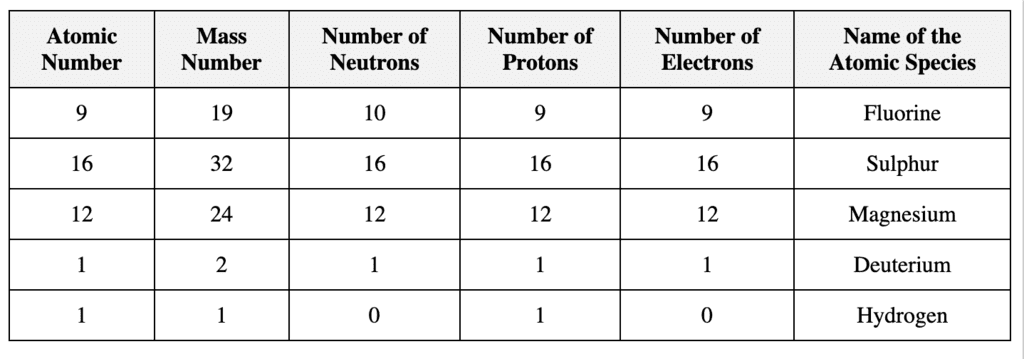
Q19. Complete the following table.
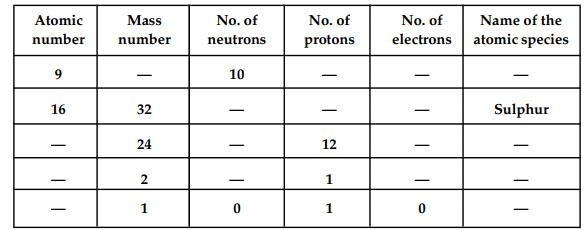 Ans:
Ans:
Atomic number(Z) =Number of protons
Mass number = Number of neutrons + atomic number
=> Mass number(A) = Number of neutrons + number of neutrons
|
84 videos|478 docs|60 tests
|
FAQs on NCERT Solutions for Class 9 Science Chapter 4 - Structure of the Atom
| 1. What is the structure of an atom? |  |
| 2. What is the charge of protons, neutrons, and electrons? |  |
| 3. How are the atomic number and mass number defined? |  |
| 4. What is the significance of isotopes in chemistry? |  |
| 5. How does the arrangement of electrons in an atom affect its chemical properties? |  |
















