Alkanes: Preparation, Properties & Conformations | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| What are Alkanes? |

|
| General Method of Preparation |

|
| Physical Properties of Alkanes |

|
| Chemical Properties of Alkanes |

|
| Conformations |

|
| Some Important Questions |

|
What are Alkanes?
- Alkanes are a group of compounds made up of carbon and hydrogen atoms connected by single covalent bonds. They are called saturated hydrocarbons and follow a pattern with the molecular formula CnH2n+2.
- Alkanes are the most basic type of hydrocarbons, consisting solely of carbon and hydrogen atoms. In these compounds, each carbon atom forms four bonds, while each hydrogen atom forms one.
- To represent them more conveniently, chemists often use line-angle formulas, which are quicker to draw than condensed structural formulas. Additionally, structural formulas for alkanes can be expressed in a more condensed manner.
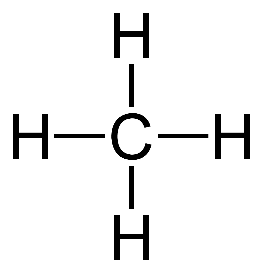 Simplest Alkane: Methane(CH4)
Simplest Alkane: Methane(CH4)
Nomenclature of Saturated Hydrocarbons
Nomenclature is a system of terms or rules that are used for forming these terms or names in a distinct field of science and arts. In simple terms, it is an assignment of names to organic compounds.
Saturated hydrocarbons are organic compounds that consist of carbon and hydrogen single bonds. In these compounds, there is the maximum number of hydrogen atoms present for every carbon atom. For example Alkanes.
The saturated hydrocarbons are named according to the following rules:
- Longest Chain Rule: The parent chain of the compound is considered the longest chain of carbon atoms. This chain can either be straight or of any other shape.
- Lowest Set of Locants: The numbering of the carbon atoms starts from the end which gives the lowest number to the carbon atom carrying the substituent.
- Presence of Same Substituent More Than Once: Prefixes such as di, tri, etc are given to the substituents which are present twice, thrice respectively on the parent chain.
- Naming Different Substituents: If more than one substituent is present then the substituents are arranged in their alphabetical order.
- Naming Different Substituents At Equivalent Positions: If two different substituents are present in the same position from the two ends then the substituents are named so that the substituent which comes first in the alphabetical order gets the lowest number.
- Naming The Complex Substituents: Naming of the complex substituent is done when the substituent on the parent chain has a branched structure (i.e. complex structure). These substituents are named as a substituted alkyl group and the carbon atom of this substituent attached to the parent chain is numbered as 1. The name of this type of substituent is written in brackets.
Let us understand it with the help of an example: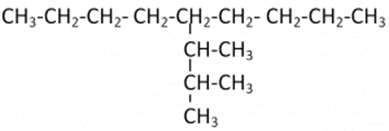 In this case, we have 9 carbon atoms in the straight chain. 5th Carbon atom from both ends of the straight chain consists of a substituent having 3 carbon chains. On the first two carbon atoms of the substituent group, there is one additional carbon atom attached.
In this case, we have 9 carbon atoms in the straight chain. 5th Carbon atom from both ends of the straight chain consists of a substituent having 3 carbon chains. On the first two carbon atoms of the substituent group, there is one additional carbon atom attached.
Now if we consider this as a new parent chain, it has a substituent which has one additional carbon each. For naming them we will first number the parent chain. In this case, we have 9 carbon atoms in a straight chain which is also the parent chain. Then we find that the substituent is on the fifth position.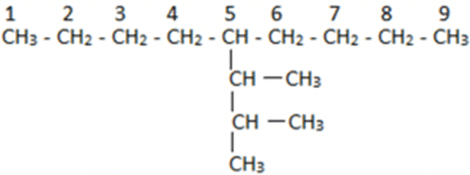 Now taking the substituent we will observe that we have 3 substituent carbons and out of these three, two substituents have additional carbons attached. We find that the longest chain in this can be the first four carbon atom chain but this is wrong as the last carbon is not attached to the parent chain.
Now taking the substituent we will observe that we have 3 substituent carbons and out of these three, two substituents have additional carbons attached. We find that the longest chain in this can be the first four carbon atom chain but this is wrong as the last carbon is not attached to the parent chain.
So we will consider only three carbon atom chains as the main chain. Thus it can be named as propane and on the first and second position, we have methyl group. We can write the name as 1-2 Dimethyl propane, but it will be written as 1-2 Dimethyl propyl as it is a substituent group.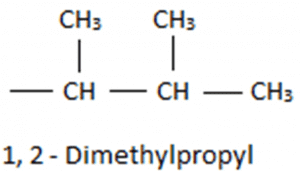 Now taking the substituent with the parent chain we will get 5-(1-2-Dimethyl Propyl) and as the parent chain has 9 carbon atoms so, it will be named as nonane. Thus, the final name of the compound will be 5-(1-2-Dimethyl Propyl)nonane.
Now taking the substituent with the parent chain we will get 5-(1-2-Dimethyl Propyl) and as the parent chain has 9 carbon atoms so, it will be named as nonane. Thus, the final name of the compound will be 5-(1-2-Dimethyl Propyl)nonane.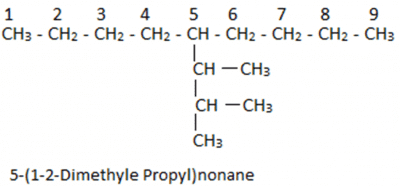
General Method of Preparation
By Catalytic Reduction of Alkenes and Alkynes
- When dihydrogen gas is introduced to alkenes and alkynes, with the help of catalysts like platinum, palladium, or nickel, it combines to form alkanes. This chemical transformation is known as hydrogenation.
- The metals, such as platinum and palladium, attract dihydrogen gas to their surfaces and make the hydrogen-hydrogen bond active. Platinum and palladium can facilitate this reaction at room temperature, but nickel catalysts need higher temperatures and pressures for the process to occur.
- R - C º ≡ C - R'
R - CH2 - CH2 - R'
- R - CH = CH - R'
R - CH2 - CH2 - R'
- Hydrogenation is exothermic, qualitative and during the hydrogenation, total heat evolved to hydrogenate one mole of an unsaturated compound is called heat of hydrogenation. The heat of hydrogenation is the measurement of the stability of isomeric alkenes.
Stability of alkene
From Alkyl Halide
1. From Organometallic Compound
(a) By Wurtz Reaction:
- 2R - X + 2Na R → R + 2NaX
- R - X + R' - X
R - R, R- R', R' - R'
- Mechanism: Two mechanisms are suggested
(i) Ionic mechanism:
2Na2Na+ + 2e-
(ii) Free radical mechanism:
Na
Note:
- The alkyl halide should be 1º or 2º. With 3º R - X, SN2 and free radical coupling are not possible due to steric hindrance so in that case elimination or disproportion is possible.
- In the ionic mechanism, alkyl sodium
gives
strong base as well as a nucleophile which gives SN2 with R - X. Ether should be dry otherwise, if moisture is present then
forms R - H instead of R - R with H2O.
(b) By Grignard Reaction:
(c) By Corey-House Alkane Synthesis:
- Mechanism:
R2CuLi is the source of
R2 CuLi does not react with -NO2, - CN, > C = O etc. - Example:
, if C is CH3 - CH2 - (CH2)5 - CH3, then what is Y.
Ans: CH3 - (CH2)6 - Br
(d) By Frankland's Reagent:
- R - X Zn R - X
R - R + Zn X2
- Mechanism:
2. By Reduction of Alkyl Halides
(a) With Metal-Acid:
Reducing agent: Zn / acid, Zn - Cu / H2O or Zn - Cu acid, Zn - Cu / C2H5OH, Na - Hg/acid, Al - Hg / H2O etc.- Mechanism:
(b) With Metal Hydrides:
- TPH (Ph3SnH) : It reduces 1º, 2º & 3º R - X
R - XR - H
- NaBH4:
,
Using Red P & HI
- Red P & HI is a strong reducing agent.
- R - COOH
R - CH3
R - CH3
R - CH3
- R - X
R - H
- R - OH
R - H + H2O
Decarboxylation
- Fatty acids are a good source of hydrocarbon, correction, heating of sodium salt of carboxylic acid (R - COONa) with soda lime (NaOH - CaO) gives hydrocarbon, which is known as decarboxylation (e.g. replacement of - COOH group by -H).
- Decarboxylation also takes place on heating only, when the compound is gem dicarboxylic acid or there is a keto group or double bond on ß carbon.
- Example:
, What are A and B?
Ans: A is
Question. Try this:
A,
Write the structure of A and mention its stereochemistry?
Kolbe's Electrolysis
- When you electrolyze a water solution containing the sodium or potassium salt of a carboxylic acid, it produces an alkane with an even number of carbon atoms at the anode.
- 2RCOOK + 2HOH
R-R + 2CO2 + H2 + 2KOH
- Example: 2CH3 - COOK + 2H2O
CH3-CH3 + 2CO2 + H2 + 2KOH.
If n is the number of carbon atoms in the salt of carboxylic acid, the alkane formed has 2(n - 1) carbon atoms.
Reduction of Aldehydes and Ketones
1. By Clemenson's reduction (with Zn - Hg / conc. HCl):
- R - CHO
RCH3 H2O
RCH2R' H2O
- Example:
CH3 - CHOCH3CH3 H2O
CH3CH2C2H5 H2O
- Clemenson's reduction is not used for a compound that has an acid-sensitive group.
2. By Wolf-Kishner reduction (with NH2NH2 / KOH):
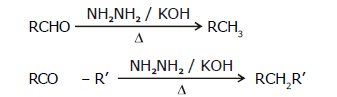
- Wolf-Kishner reduction is not used for compounds that have base-sensitive groups.
Physical Properties of Alkanes
1. Alkanes are colourless and odourless.
2. They possess weak Vander Waal's force of attraction. Apart from weak Vander Waal's forces, London forces, Dispersion forces, and weak intermolecular forces act between the molecules of alkanes.
3. Alkanes having 1-4 carbon atoms are gases, then from 5-17 carbon atoms are liquid and alkanes having 18 or more carbon atoms are solid at 298K.
4. Structure of alkanes: In alkanes, all the carbon atoms are sp3 hybridised which mean that they form four sigma bonds with either carbon or hydrogen atoms. Their general formula is CnH2n+2.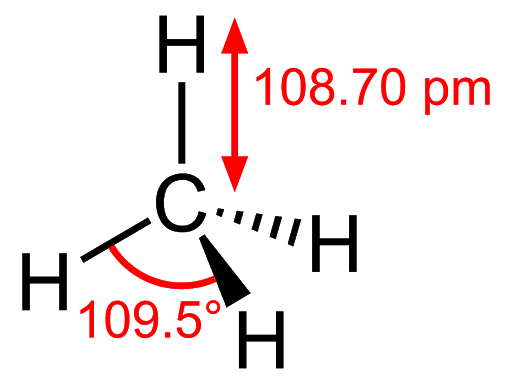
5. Boiling & Melting Point: Shorter chain alkanes have low melting and boiling points but as the number of carbon atoms in the chain increases melting and boiling point rise.
(a) Boiling Point: It increases with the increasing molecular weight as the Vander Waal's force increases with the increasing molecular weight. Straight-chain alkanes have a higher boiling point than their structural isomer.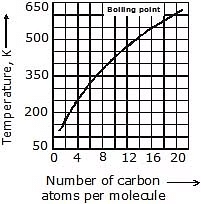
(b) Melting Point: It also increases with increasing molecular weight because it is difficult to break the intermolecular forces of attraction between higher alkanes as they are generally solids. Even-numbered alkanes have a better packing in the solid phase than the odd ones as they form a well-organised structure which is difficult to break hence even-numbered alkanes have a higher melting point than odd-numbered ones.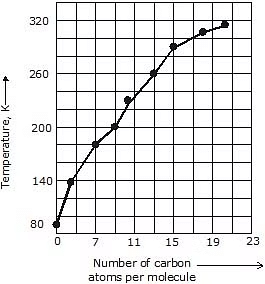
6. Solubility:
In keeping with the popular rule "like dissolves like" hydrocarbons are insoluble in polar solvent like water because they are predominantly non-polar in nature.
(a) Alkanes are generally nonpolar molecules because of the covalent bonds between C-C and C-H and also because of the very small difference between electronegativities of carbon and hydrogen.
(b) We know that polar molecules are soluble in polar solvents and nonpolar molecules are soluble in nonpolar solvents, generally, so this implies alkanes are insoluble in water or hydrophobic in nature.
(c) When a non-polar alkane is added to a polar solvent, the water molecules are attracted to each other and alkane molecules do not attract each other.
(d) In organic solvents, they are soluble because the energy required to overcome existing Vander Waal's forces and to generate new Vander Waal's forces is quite comparable.
7. Density:
The densities of alkanes increase with increasing molecular weight but become constant at about 0.8 g cm-3. This means that all alkanes are lighter than water so they floats over water. Alkanes have a lower density than water, they float on water. Density increases with an increase in molecular mass.
Chemical Reaction of Alkanes
Characteristic reaction of alkanes are free radical substitution reaction, these reactions are generally chain reactions which are completed mainly in three steps.
- Chain initiation
- Chain propagation
- Chain termination
Examples of free radical substitution reaction →
R - H + X2 R - X + HX
Exp.
When equimolar amount of methane and Cl2 are taken, a mixture of four possible products are formed, but if we take excess of CH4 then yield of CH3Cl will be the major product.
Reactivity of X2 : F2 > Cl2 > Br2 > I2
Reactivity of H : 3ºH > 2ºH > 1ºH
Alkanes reacts so vigorously with F2 that, even in the dark and at room temp, reactant diluted with an inert gas.
Iodination is a reversible reaction since HI formed as a by-product is a strong reducing agent and reduces alkyl iodide back to alkane. Hence iodination can be done only in presence of strong oxidizing agent like HIO3, HNO3 or HgO.
R - H + I2 R - I + HI
HI + HIO3 H2O + I2
Mechanism of halogenation of CH4 →
(i) Chain initiation → It is an endothermic step.
X2
(ii) Chain propagation →
(iii) Chain termination → It is always exothermic.
Each photon of light leaves one chlorine molecule to form two chlorine radicals, each chlorine atom starts a chain and on an average each chain contains 5000 repetitions of the chain propagating cycle so about 10,000 molecules of CH3Cl are formed by one photon of light.
Some reagent affects the rate of halogenation:
Example: In the given ways which is feasible
Q.4 Which of the following reaction has zero activation energy
(A)
(B) Cl2 2 Cl
(C)
(D)
Q.5 If the Eact for a forward reaction is given
the Eact for backward reaction will be
(A) 1 kcal
(B) 4 kcal
(C) -4 kcal
(D) 3 kcal
Halogenations of higher alkanes:
(i)
(ii)
(iii)
(iv)
(v)
Relative amounts of the various isomers differ remarkably depending upon the halogen used from the above reaction, it is observed that chlorination gives mixture in which no isomer greatly dominates while, bromination gives mixture in which one isomer dominates greatly (97% - 99%).
Factors determining the relative yields of the isomeric products.
(i) Probability factor → This factor is based on the number of each kind of H atom in the molecule.
(ii) Reactivity of hydrogen → The order of reactivity is 3º > 2º > 1º
1.4.2 Aromatisation:
1.4.3 Combustion : (i.e. complete oxidation)
O2
nCO2 + (n+1) H2O (DHcombustion = -ve)
O2
xCO2 +
H2O
C5H12 + 8O2 5CO2 + 6H2O
Heat of combustion: Amount of heat i.e. liberated when 1 mole of hydrocarbon is completely burnt into CO2 & H2O.
Combustion is used as a measurement of stability. More branched alkanes are more stable and have lower heat of combustion.
e.g. (I) CH3 - CH2 - CH2 - CH3
(II)
stability : II > I
DHcomb. : I > II
More branched alkane has more no. of primary C - H bonds. (therefore it has more bond energy).
Homologous : Higher homologous have higher heat of combustion.
Isomers : Branched isomer has lower heat of combustion.
(i) Initiators → They initiate the chain reaction. Initiators are R2O2, Perester's etc.
R - O - O - R
(ii) Inhibitors → A substance that slow down or stop the reaction are known as inhibitors. For example, O2 is a good inhibitor.
All reactive alkyl free radicals are consumed so reaction stops for a period of time.
Relative reactivity of halogen toward methane→
Order of reactivity is F2 > Cl2 > Br2 > I2 which can be explained by the value of ΔH (energy change).
Steps of halogenation, value of ΔH for each step. (Kcal/mole)
Ex.3 Explain why the chain initiating step in thermal chlorination of CH4 is
Cl2 and not CH4
Ans. Because Eact of Cl2 is less than Eact of CH4.
Ex.4 Chlorination of CH4 involves following steps :
(i)
(ii)
(iii)
Which of the following is rate determining?
(A) Step (i)
(B) Step (ii)
(C) Step (iii)
(D) Step (ii) and (iii) both
Ans. (B)
Reactivity of hydrogen 3º > 2º > 1º
Because formation of alkyl free radical is Rate determining step so, as H is more reactive which produce more stable free radical (less Eact).
order of stability of Free Radical.→
Chemical Properties of Alkanes
Alkanes are quite inert substances with a highly stable nature.
Their inactiveness has been explained as:
- In alkanes, all the C-C & C-H bonds are stronger sigma bonds and are not influenced by acids, alkalies, or oxidants under ordinary conditions.
- The C-C (completely non-polar) & C-H (weak polar) bonds in alkanes- are practically non-polar because of the small electronegativity difference in C (2.6) and H (2.1).
- Thus polar species i.e., electrophiles or nucleophiles are unable to attack these bonds under ordinary conditions.
- Despite a less reactive nature, alkanes show some characteristic reactions.
Oxidation Reactions of Alkane
Oxidation of alkanes gives different products under different conditions.
1. Complete oxidation or combustion:
- Alkanes burn readily with non-luminous flame in the presence of air or oxygen to give CO2 & water along with the evolution of heat. Therefore, alkanes are used as fuels.
- CnH2n+2 + [(3n+1)/2] O2 → n CO2 + (n+1) H2O; ΔH = -ve
- Example: CH4 + 2 O2 → CO2 + 2 H2O; ΔH = -ve
2. Incomplete oxidation:
- The incomplete oxidation of alkanes in a limited supply of air gives carbon black and carbon monoxide.
- 2 CH4 + 3 O2 → 2 CO + 4 H2O
- CH4 + O2 → C (carbon black) + 2 H2O
3. Catalytic oxidation:
- Lower alkanes are easily converted to alcohols and aldehydes under controlled catalytic oxidation.
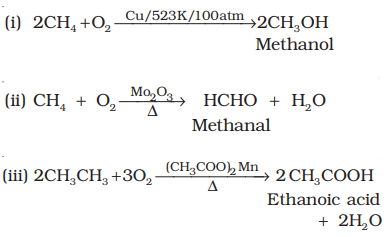
- Higher alkanes on oxidation in the presence of manganese acetate give fatty acids.

4. Chemical Oxidation:
- Tertiary alkanes are oxidized to tertiary alcohols by KMnO4.
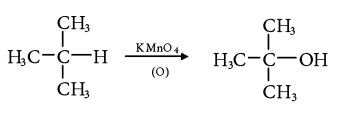
Substitution Reactions of Alkanes
Substitution in alkanes shows a free radical mechanism. For mechanism see free radical substitution.
Following substitution reactions in alkanes are noticed:
1. Halogenation of Alkanes
- Chlorination may be brought about by photoirradiation, heat, or catalysts, and the extent of chlorination depends largely on the amount of chlorine used.
- A mixture of all possible isomeric monochlorides is obtained, but the isomers are formed in unequal amounts, due to differences in reactivity of primary, secondary, and tertiary hydrogen atoms.
- The order of ease of substitution is:
Tertiary Hydrogen > Secondary Hydrogen > Primary Hydrogen - Chlorination of isobutane at 300o C gives a mixture of two isomeric monochlorides.
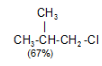
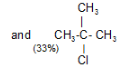
The tertiary hydrogen is replaced by about 4.5 times as fast as primary hydrogen.
Bromination is similar to chlorination, but not so vigorous.
Iodination is reversible, but it may be carried out in the presence of an oxidising agent such as HIO3, HNO3 etc., which destroys the hydrogen iodide as it is formed and so drives the reaction to the right,
Example:
CH4 + I2 → CH3I + HI
5 HI + HIO3 → 3 I2 + H2OIodides are more conveniently prepared by treating the chloro- or bromo-derivative with sodium iodide in methanol or acetone solution.
i.e. RCl + NaI—→ RI + NaCl (in the presence of acetone).This reaction is possible because sodium iodide is soluble in methanol or acetone, whereas sodium chloride and sodium bromide are not. This reaction is known as Conant Finkelstein reaction.
Direct fluorination is usually explosive; special conditions are necessary for the preparation of the fluorine derivatives of the alkanes.
RH + X2 —→ RX + HX
(Reactivity of X2: F2 > Cl2 > Br2; I2 does not react)Mechanism of methane chlorination:
(a) Initiation Step:
Cl : Cl —→ 2Cl· ; ΔH = +243 kJ mol-1
The required enthalpy comes from ultraviolet (UV) light or heat.
(b) Propagation Step:
(i) H3C : H + Cl· → H3C· + H : Cl ; ΔH = -4 kJ mol-1 (rate-determining)
(ii) H3C· + Cl : Cl → H3C : Cl + Cl· ; ΔH = -96 kJ mol-1
(iii) The sum of the two propagation steps in the overall reaction,
CH4 + Cl2 → CH3Cl + HCl; ΔH= -100 kJ mol-1
(c) Terminating Step:
In propagation steps, the same free radical intermediates (here Cl· and H3C·) were being formed and consumed. Chains terminate on those rare occasions when two free-radical intermediates form a covalent bond.
Cl· + Cl· → Cl2
H3C· + Cl· → CH3 : Cl
H3C· + ·CH3 → H3C : CH3
Inhibitors stop chain propagation by reacting with free radical intermediates.
Example: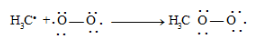
In more complex alkanes, the abstraction of each different kind of H atom gives a different isomeric product. Three factors determine the relative yields of isomeric products:
(i) Probability Factor: This factor is based on the number of each kind of H atom in the molecule.
Example: In CH3CH2CH2CH3, there are six equivalent 1° H’s and four equivalent 2° H’s. The ratio of abstracting a 1° H is 6 to 4 or 3 to 2.
(ii) Reactivity of H.: The order of reactivity of H is 3° > 2° > 1°.
(iii) Reactivity of X.: The more reactive Cl. is, it is less selective and more influenced by the probability factor. The less reactive Br. , it is more selective and less influenced by the probability factor, as summarized by the Reactivity-Selectivity Principle. If the attacking species is more reactive, it will be less selective, and the yields will be closer to those expected from the probability factor.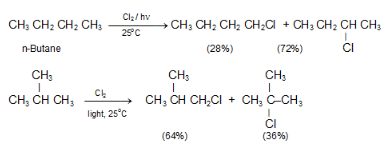
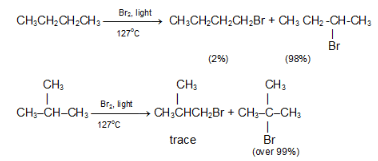
In the chlorination of isobutane abstraction of one of the nine primary hydrogens leads to the formation of isobutyl chlorides, whereas abstraction of single tertiary hydrogen leads to the formation of tert-butyl chloride.
The probability of favourable formation of isobutyl chloride is of the ratio 9:1. But the experimental results show the ratio roughly to be 2:1 or 9:4.5. Evidently, about 4.5 times as many collisions with the tertiary hydrogen are successful as collisions with the primary hydrogen. The Eact is less for the abstraction of tertiary hydrogen than for the abstraction of primary hydrogen.The rate of abstraction of hydrogen atoms is always found to follow the sequence 3º > 2º > 1º.
Example: At room temperature, the relative rate per hydrogen atom is 5.0 : 3.8 : 1.0. Using these values we can predict quite well the ratio of isomeric chlorination products from a given alkane.
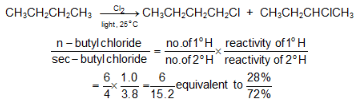
Despite these differences in reactivity, chlorination rarely yields a great excess of any single isomer.The same sequence of reactivity, 3° > 2° > 1°, is found in bromination, but with enormously larger reactivity ratios.
Example: At 127 °C, the relative rates per hydrogen atom are 1600:82:1. Here, differences in reactivity are so marked as vastly outweigh probability factors. Hence bromination gives a selective product.
In bromination of isobutane at 127 ºC,
Hence, tert-butyl bromide happens to be the exclusive product (over 99%).
2. Nitration
- Replacement of the H atom of alkane by the -NO2 group is known as nitration. Nitration of an alkane is made by heating vapours of alkanes and HNO3 at about 400 ºC to give nitroalkanes. This is also known as vapour phase nitration.
CH4(g) + HNO3(g)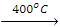 CH3NO2 + H2O
CH3NO2 + H2O - During nitration, C-C bonds of alkanes are also decomposed due to the strong oxidant nature of HNO3 to produce all possible nitroalkanes.
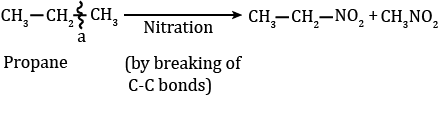
- The nitration of alkane also shows the order: Tertiary H > Secondary H > Primary H > methane
- The nitration of alkanes follows a free-radical mechanism:
HONO2 HO· + ·NO2
HO· + ·NO2
C3H7-H + HO· → C3H7· + H2O
C3H7· + ·NO2 → C3H7NO2
3. Sulphonation:
- Replacement of the H atom of alkane by -SO3H is known as sulphonation. Lower normal alkanes are not sulphonate, but higher normal alkanes show sulphonation (hexane onwards) when heated with oleum (i.e., conc. H2SO4) at 400 ºC.
C6H14 + H2SO4 → C6H13SO3H + H2O - Lower members are sulphonated in vapour phase sulphonation.
- The reactivity order for sulphonation: T.H. > S.H. > P.H.
- Thus isobutene is easily sulphonated as it contains a tertiary hydrogen atom.
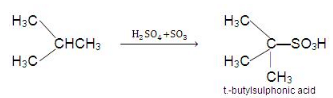
- Sulphonation of alkanes also follows free-radical mechanism:
HOSO3H
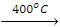 HO· + ·SO3H
HO· + ·SO3HC3H13-H + ·OH → C6H13· + H2O
C3H13· + ·SO3H → C6H13SO3H
4. Isomerization
- The process of conversion of one isomer into another is known as isomerization. Straight-chain alkanes on heating with AICI3 + HCI at about 200 ºC and 35 atm pressure are converted into branched-chain alkanes.
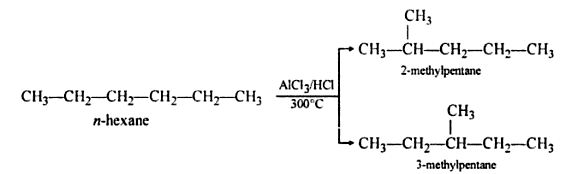
5. Aromatization
- The process of conversion of an aliphatic compound into an aromatic compound is known as aromatization. Alkanes having six to 10 carbon atoms are converted into benzene and its homologues at high pressure and temperature in the presence of a catalyst.
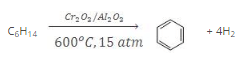
6. Dehydrogenation
- Alkanes are dehydrogenated on heating in the presence of a catalyst to produce corresponding alkenes.

7. Pyrolysis
- The decomposition of a compound on heating in the absence of air is known as pyrolysis. The phenomenon of pyrolysis of an alkane is also known as cracking.
- Alkane vapours on passing through a red hot metal tube in the absence of air decompose into simpler hydrocarbons.
- The product formed during cracking depends upon:
(a) Nature of alkane
(b) Temperature and Pressure
(c) Presence or absence of a catalyst - The ease of cracking in alkanes increases with an increase in molecular weight and branching in an alkane.
- The fission of C-C bonds produces alkanes and alkenes whereas fission of C-H bonds produces alkene and hydrogen.
- The presence of Cr2O3, V2O2, MoO3 catalyses C-H bond fission and the presence of SiO2, AI2O3, and ZnO catalyses C-C bond fission.
- The no. of products obtained during cracking increases with an increase in the molecular weight of the alkane undergoing cracking.
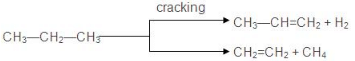
- Cracking has an important role in the petroleum industry. Higher alkanes are converted into lower ones (petrol C6 to C11) by cracking.
8. Aromatisation
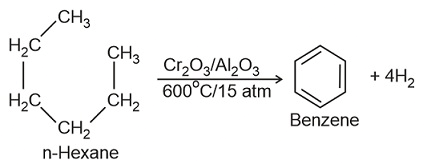
Conformations
- In organic chemistry, it's observed that you can rotate a single bond in a molecule to rearrange the atoms, and this doesn't alter the compound's chemical formula.
- Therefore, by rotating a single bond, you can generate isomers of a compound. The various atom arrangements resulting from these bond rotations are called conformations or conformers or rotamers.
- Alkanes have C-C sigma (σ) bonds and rotation about C-C single bond is allowed. This rotation results in different spatial arrangements of atoms in space which can change into one another.
- It's important to note that rotation around a C-C single bond is not entirely unrestricted. There's a slight energy barrier, ranging from 1 to 20 kJ mol–1, caused by weak repulsive forces between neighbouring bonds. This type of repulsive interaction is referred to as torsional strain.
Conformations of ethane
- Ethane (C2H6) is a molecule with a carbon-carbon single bond, and each carbon is connected to three hydrogen atoms.
- If you visualize ethane using a ball-and-stick model and rotate one carbon atom around the C-C axis while keeping the other stationary, you get an infinite number of spatial arrangements of hydrogen atoms.
- These different arrangements are called conformational isomers or conformers, and ethane can have an infinite number of them.
- There are two extreme cases: the eclipsed conformation, where hydrogen atoms are as close together as possible, and the staggered conformation, where hydrogens are as far apart as possible.
- Any other arrangement between these extremes is known as a skew conformation.
- It's important to note that in all conformations, the bond angles and lengths remain the same.
- Eclipsed and staggered conformations can be represented using Sawhorse and Newman projections.
In simple words, when you rotate one carbon atom in ethane around the other, you can create many different arrangements of hydrogen atoms.
The extremes are when hydrogens are very close together (eclipsed) or as far apart as possible (staggered), with various in-between arrangements called skew or gauche conformations. Even though the shapes change, the bond angles and lengths stay the same. We can represent these extremes using Sawhorse and Newman projections.
Sawhorse Projections
- This projection is a way to look at a molecule along its molecular axis.
- The central C-C bond is represented by a slightly extended straight line on paper.
- The upper end of this line is tilted slightly to the right or left.
- The front carbon appears at the lower end of the line, while the rear carbon is at the upper end.
- Each carbon is depicted with three lines extending from it, representing three hydrogen atoms.
- These lines are inclined at an angle of 120° to each other.
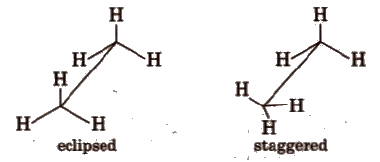 Sawhorse Projections
Sawhorse Projections
Newman Projections
- This projection involves looking at the molecule directly along the C-C bond.
- The carbon closer to the viewer is symbolized by a point.
- Three lines extending at 120° angles from each other represent the three hydrogen atoms attached to the front carbon.
- The rear carbon (farther from the viewer) is depicted as a circle.
- Shorter lines extending at 120° angles represent the three hydrogen atoms attached to the rear carbon.
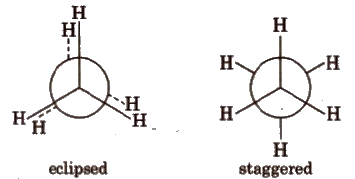 Newman Projections
Newman Projections
Relative Stability of Conformations
- In the staggered conformation of ethane, the electron clouds of carbon-hydrogen bonds are maximally separated, resulting in minimal repulsive forces, low energy, and high stability.
- Transitioning to the eclipsed conformation brings electron clouds of carbon-hydrogen bonds closer, increasing repulsions, and energy, and decreasing stability.
- The repulsive interaction between electron clouds, known as torsional strain, depends on the angle of rotation (dihedral angle) around the C-C bond.
- The staggered conformation is more stable than the eclipsed, making it the preferred state for the molecule.
- Despite a small energy difference of about 12.5 kJ mol–1 between extreme forms, ethane easily overcomes this barrier at ordinary temperatures through collisions.
- For practical purposes, rotation around the carbon-carbon single bond in ethane is almost free due to the molecule gaining sufficient thermal or kinetic energy.
- Separating and isolating different conformational isomers of ethane has not been possible.
Some Important Questions
Q1: The number of possible isomeric products formed when 3-chloro-1-butene reacts with HCl through carbocation formation is __________. [2023]
Ans: 4
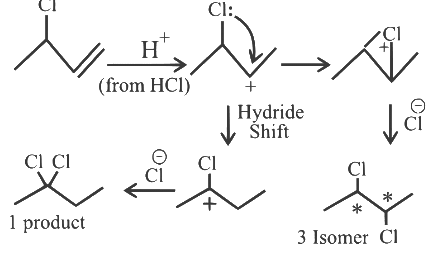
Total Possible Isomeric product = 1 + 3 = 4
Q2: But-2-yne is reacted separately with one mole of Hydrogen as shown below :
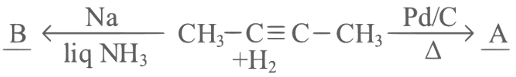 A. A is more soluble than B.
A. A is more soluble than B.
B. The boiling point & melting point of A are higher and lower than B respectively.
C. A is more polar than B because dipole moment of A is zero.
D. Br2 adds easily to B than A.
Identify the incorrect statements from the options given below :
(a) A and B only
(b) A, C & D only
(c) B, C & D only
(d) C and D only [2023]
Ans: (d)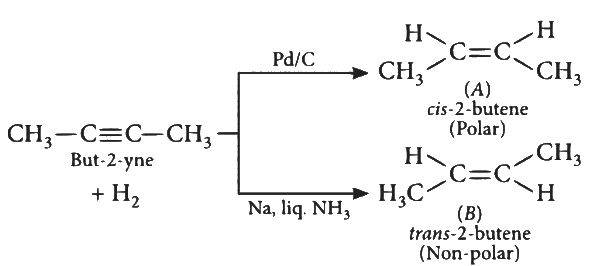
cis-2-butene is more polar that is why it's solubility is more than trans-2-butene.
∴ A is correct.
cis-2-butene has a lower melting point than trans-2-butene as trans-form has compact packing crystal lattice. cis-2-butene is polar so, it has higher boiling point than trans-form.
∴ B is also correct.
cis-2-butene is more polar but dipole moment of cis-2-butene(A) is not zero.
∴ C is incorrect.
'Cis' structure is less stable than 'Trans" structure. Less stable means more reactive. So Cis - But-2-ene(A) will react more easily with Br2.
∴ D is also incorrect.
Q3: What will be the major product of following sequence of reactions? [2022]
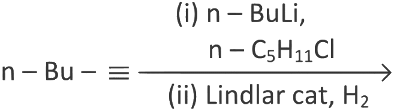
(a) 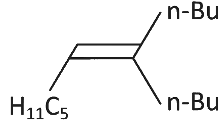
(b) 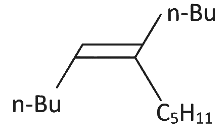
(c) 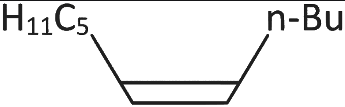
(d) 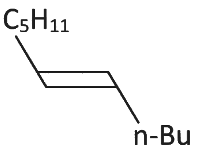
Ans: (c)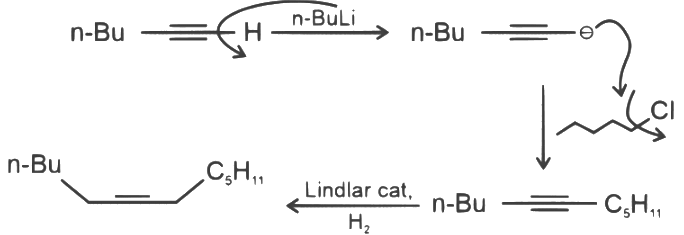
Hence correct option is (C).
|
119 videos|338 docs|74 tests
|
FAQs on Alkanes: Preparation, Properties & Conformations - Chemistry Class 11 - NEET
| 1. What are some common methods of preparing alkanes? |  |
| 2. What are some physical properties of alkanes? |  |
| 3. What are some common chemical properties of alkanes? |  |
| 4. What are conformations in alkanes? |  |
| 5. What are some important questions related to alkanes that may be asked in the NEET exam? |  |





















