Atomic Models: Dalton, Thomson & Rutherford | Chemistry Class 11 - NEET PDF Download
The topic of Atomic Models in Class 11 Chemistry deals with the various scientific theories and models proposed by scientists to explain the structure and behavior of atoms. In this document, we will delve into these models to gain a better understanding of atomic structure.
Dalton's Atomic Model
In 1808, a British school teacher named John Dalton put forward the first solid scientific explanation of how matter is made up. His idea, known as Dalton's atomic theory, considered the atom as the tiniest building block of all substances.
 Dalton's Atomic Theory
Dalton's Atomic Theory
Postulates of Dalton's Atomic Theory:
- Tiny Building Blocks:
Everything around us is made up of extremely small, indivisible particles known as atoms. - Uniformity Within Elements:
All atoms of a particular substance (element) are the same in terms of mass, size, and other characteristics. However, atoms of different elements are unique and differ in these properties. - Indestructible Particles:
Atoms cannot be created or destroyed, and they cannot be split into smaller parts. - Combining in Fixed Ratios:
Atoms of different elements can join together in specific whole-number ratios to create compounds. - Chemical Changes through Atom Rearrangement:
Chemical reactions involve the rearrangement, combination, or separation of atoms.
Limitations of Dalton's Atomic Theory:
- Misses Subatomic Particles:
Dalton thought atoms were the smallest bits, but later discoveries revealed smaller particles like protons, electrons, and neutrons. - Overlooks Isotopes:
Dalton assumed all atoms of an element have the same mass, but isotopes (variants of elements) can have different masses. - Not Aware of Isobars:
Dalton suggested that different elements must have different masses, but isobars, where different elements share the same mass number, were later discovered. - Complex Ratios in Compounds:
Some compounds, especially complex organic ones, don't always follow simple whole-number ratios of atoms in Dalton's theory. For example, sugar (C11H22O11). - No Explanation for Allotropes:
The theory can't clarify the distinct properties of different forms of the same element, like diamond and graphite, both made of carbon.
What is Thomson's Model of Atom?
Thomson's Model of Atom is also known as the Plum Pudding Model, which was proposed by J.J. Thomson in 1904. The model is based on the discovery of the electron by Thomson in 1897.
 J.J. ThomsonDescription of Thomson Model:
J.J. ThomsonDescription of Thomson Model:
- The atom consists of a positively charged sphere.
- Negatively charged electrons are embedded in the sphere.
- Electrons are distributed randomly throughout the sphere.
- The positive charge is uniformly distributed throughout the atom.
- The atom is neutral overall, with equal numbers of positive and negative charges canceling each other out.
Explanation:
- Thomson's model could explain some of the properties of atoms, such as their ability to conduct electricity and their chemical reactivity.
- The model provided an explanation for how the negatively charged electrons were held within the atom without being repelled by the positively charged protons.
- The model also provided a possible explanation for why atoms had a finite size, as the negatively charged electrons would repel each other, causing the atom to expand.
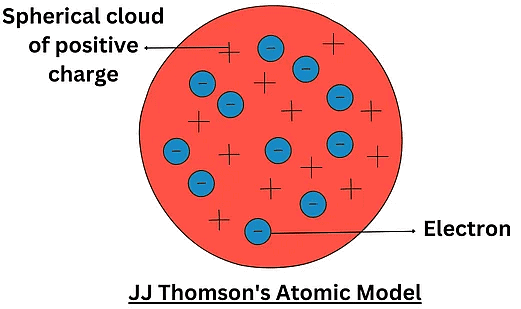 JJ Thomson Atomic Model
JJ Thomson Atomic Model
Postulates of Thomson’s Atomic Model:
- Postulate 1: An atom consists of a positively charged sphere with electrons embedded in it
- Postulate 2: An atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude
Thomson's atomic model is compared to watermelon. Where he considered watermelon seeds as negatively charged particles and the red part of the watermelon as positively charged.
Important Features & Significance:
- Thomson's Plum Pudding Model was an important step towards understanding the structure of the atom.
- The model helped scientists to understand the behavior of atoms and the role of electrons in chemical reactions.
- The model was eventually replaced by more accurate models, such as Rutherford's Nuclear Model, which proposed the existence of a small, dense, positively charged nucleus at the center of the atom.
What are the Limitations of Thomson’s Atomic Model?
While Thomson's Atomic Model provided a significant contribution to the development of atomic theory, it also had several limitations and drawbacks. Here are some of the major limitations of Thomson's atomic model:
- No concept of the nucleus: Thomson's atomic model did not account for the existence of the nucleus. He proposed that the atom was a uniform, positively charged sphere with negatively charged electrons embedded within it. This model suggested that the atom's mass was evenly distributed throughout the atom, which was later proven to be incorrect.
- No explanation of spectral lines: Thomson's model could not explain the origin of spectral lines observed in the emission spectra of various elements. This was because he believed that the electrons were free to move throughout the atom, and therefore there should be a continuous spectrum of radiation instead of the observed discrete lines.
- Failed to explain radioactivity: Thomson's model was unable to account for the phenomenon of radioactivity, which was discovered around the same time as his model was proposed. This was because his model did not include the concept of the nucleus, which is responsible for radioactive decay.
- Inaccurate assumptions about electron behavior: Thomson's model assumed that electrons were freely moving within the atom, and were held in place by electrostatic forces. However, later experiments showed that electrons are confined to specific energy levels within the atom and move in discrete orbits.
- Inability to explain isotopes: Thomson's model also failed to explain the phenomenon of isotopes, where atoms of the same element have different atomic masses due to differences in the number of neutrons in the nucleus.
Despite these limitations, Thomson's atomic model was an important step forward in our understanding of atomic structure. It was the first model to propose that atoms were made up of subatomic particles and paved the way for further research and development of atomic theory.
What is Rutherford's Model of Atom?
Rutherford's Model of Atom, also known as the Nuclear Model, was proposed by Ernest Rutherford in 1911. The model is based on the famous gold foil experiment conducted by Rutherford and his colleagues in 1909.
 Ernest Rutherford
Ernest Rutherford
Rutherford's α-particle scattering experiment Set-Up:
 Rutherford's Gold Foil Experiment
Rutherford's Gold Foil Experiment
- A thin gold foil was bombarded with α-particles emitted from a radioactive source.
- The α-particles were directed towards the gold foil and the scattered α-particles were detected using a zinc sulfide screen.
Observations of α-particle experiment: Observations of α-particle Experiment
Observations of α-particle Experiment
- Most of the α-particles passed through the foil undeflected or deflected by very small angles [less than 1º].
- Few particles were deflected through angles as large as 90º.
- Very few particles [ 1 in 20 thousand] bounce back or rejected through an angle almost equal to 180º.
- The angle of deflection increases with an increase in atomic number.
Conclusions
- Since most of the α-particles passed through the foil undeflected, therefore there must be largely empty space present in the atom.
- α-particles are positively charged and having considerable mass could be deflected by only some heavy positively charged centre by repulsion. [Rutherford named this positively charged heavy centre as nucleus].
- Since very few α-particles were thrown back, therefore, the size of the nucleus should be very small and should be rigid because α-particle can recoil back only if it undergoes direct collision with the heavy positively charged centre.
- On the basis of the scattering experiment, Rutherford proposed an atomic model or nuclear atomic model or nuclear model of atoms.
 Rutherford's Gold Foil Experiment
Rutherford's Gold Foil Experiment
Rutherford's Atomic Model
- The electrons are revolving around the nucleus in a circular path and the electrostatic force of attraction between the electrons and nucleus is balanced by the centrifugal force acting on the revolving electron.
- The -ve charge on electrons is equal to the total positive charge on the nucleus and therefore as a whole atom is electrically neutral.
- The size of the nucleus is extremely small as compared to the size of the atom.
Significance of Rtherford's Gold Foil Experiment
- The α-particle scattering experiment provided evidence for the existence of the atomic nucleus.
- The experiment helped to establish the nuclear model of the atom and paved the way for further research on the atomic structure.
- The experiment provided a basis for the development of more accurate models of atomic structure, such as Rutherford's model and Bohr's model of the atom.
 Structure of an Atom
Structure of an Atom
Radius of Nucleus
The radius of a nucleus is of the order of 10-15 m and the radius of an atom is in the order of 10-10 m.
r = r0 A1/3
A = Mass number = no. of protons no. of neutrons.
r0 = constant = 1.2 × 10-13 cm.
Distance of Closest Approach

If the α-particle is projected with some velocity v, from a very large distance then at point (1), the energy of the α-particle is: E = [1/2 x mv2] (K.E.) + 0 (P.E.)
As the particle moves closer to the nucleus of the other particle, the repulsive coulombic force increases (P.E. increases), and the velocity of the particle decreases, kinetic energy is converted into P.E.
∴ At point (2) E = K.E. + P.E. = 
[As no ext. force is acting, ∴ Total energy of the system remains constant].
velocity becomes zero at rmin distance where Coulombic potential energy of repulsion becomes equal to the initial kinetic energy.
At point (3),
q1 = z1e ; q2 = z2e
e = electronic charge = 1.6 × 10-19 C
z1 = z2 = Atomic number
Solved Example on Distance of Closest Approach
Example: Calculate the distance of closest approach to the nucleus of gold (Z = 79) foil when an alpha particle of 10 MeV is scattered through 180° from the gold foil.
Solution:
Given,
Atomic number of gold = 79
Kinetic energy = 1/2mu2 = 10 MeV = 10 × 106 = 107 eV = 107 × 1.6 × 10-19 J = 1.6 × 10-12 J
d/(1/4πϵo)=9×109N
We know, e = 1.6 × 10-19 C
To find the distance of closest approach (ro)


What are the Limitations of Rutherford's Atomic Model?
Ernest Rutherford's atomic model, also known as the nuclear model, was proposed in 1911. It proposed that the atom was mostly empty space, with a dense, positively charged nucleus at the center, surrounded by negatively charged electrons orbiting the nucleus. While this model provided a better understanding of atomic structure than Thomson's model, it also had its limitations. Here are some of the main limitations of Rutherford's atomic model:
 Drawback of Rutherford's Atomic Model
Drawback of Rutherford's Atomic Model
- No explanation of electron behavior: Rutherford's model did not explain how electrons were arranged or moved around the nucleus. It was assumed that electrons moved in circular orbits around the nucleus, but it was later discovered that electrons move in complex patterns within specific energy levels.
- Failure to explain the stability of the atom: According to classical electromagnetism, negatively charged electrons orbiting around a positively charged nucleus would gradually lose energy and spiral into the nucleus, causing the atom to collapse. Rutherford's model could not explain why this did not happen and how the atom remained stable.
- No explanation for the number of electrons in an atom: Rutherford's model did not explain the number of electrons present in an atom, or why certain elements had specific numbers of electrons in their outermost energy levels.
- Inability to explain chemical properties: Rutherford's model could not explain the chemical properties of elements, such as why some elements were reactive while others were inert.
- Ignored the wave-particle duality of electrons: Rutherford's model treated electrons as particles, ignoring their wave-like properties, which were later discovered by scientists.
Despite its limitations, Rutherford's model was a significant advancement in atomic theory and paved the way for further research and development of atomic models. It provided a new understanding of the atom's structure and set the stage for the development of more accurate atomic models.
Frequently Asked Questions (FAQs)
Q.1. Why was Thomson’s atomic model discarded?
It was discarded because he was unable to precisely account for the stability of the atom. He proposed that electrons are distributed in the atom in the same way that seeds are distributed in a watermelon or dry fruits are distributed in a Christmas pudding.
Q.2. What are the important features of Thomson's atomic model?
The following are the characteristics of the Thomson model of an atom:
(i) An atom is made up of a positively charged sphere in which electrons are embedded.
(ii) The magnitudes of the negative and positive charges are the same. As a result, the atom is electrically neutral as a whole.
Q.3. Which model best explains the atom’s neutrality?
According to Thomson’s atomic model, an atom is made up of a positively charged sphere into which negatively charged electrons are implanted. Because electrons and protons have the same magnitude, an atom as a whole is electrically neutral.
Q.4. To which fruit has Thomson’s model been compared to?
The model was compared to a watermelon because the red edible part of a watermelon was compared to the sphere with a positive charge, and the black seeds filling the watermelon resembled the electrons inside the sphere.
Q.5. What was missing from Thomson’s atomic model?
His model lacked a nucleus, protons, and neutrons.
Q.6. What was the specialty of Rutherford’s atomic model?
Rutherford was the first to determine the presence of a nucleus in an atom. He bombarded α-particles on a gold sheet, which made him encounter the presence of positively charged specie inside the atom.
Q.7. What is Rutherford’s atomic model?
Rutherford proposed the atomic structure of elements. He explained that a positively charged particle is present inside the atom, and most of the mass of an atom is concentrated over there. He also stated that negatively charged particles rotate around the nucleus, and there is an electrostatic force of attraction between them.
Q.8. What are the limitations of Rutherford’s atomic model?
Rutherford failed to explain the arrangement of electrons in an atom. Like Maxwell, he was unable to explain the stability of the atom.
Q.9. What kind of experiment did Rutherford’s perform?
Rutherford performed an alpha scattering experiment. He bombarded α-particles on a gold sheet and then studied the trajectory of these α-particles.
Q.10. What was the primary observation of Rutherford’s atomic model?
Rutherford observed that a microscopic positively charged particle is present inside the atom, and most of the mass of an atom is concentrated over there.
|
172 videos|306 docs|152 tests
|
FAQs on Atomic Models: Dalton, Thomson & Rutherford - Chemistry Class 11 - NEET
| 1. What is Thomson's model of the atom? |  |
| 2. What are the limitations of Thomson's atomic model? |  |
| 3. What is Rutherford's model of the atom? |  |
| 4. What are the limitations of Rutherford's atomic model? |  |
| 5. How does Dalton's atomic model differ from Thomson's and Rutherford's models? |  |

|
Explore Courses for NEET exam
|

|