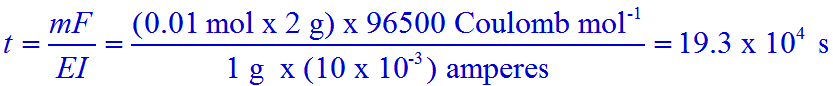Electrolytic Cells, Electrolysis & Products of Electrolysis | Chemistry Class 12 - NEET PDF Download
What is Electrochemistry?
Electrochemistry focuses on generating electricity from spontaneous chemical reactions and using electrical energy to drive non-spontaneous reactions.- It’s significant both theoretically and practically, producing metals and chemicals like NaOH and chlorine.
- Batteries and fuel cells use these principles to convert chemical energy to electricity, offering eco-friendly energy solutions.
- Electrochemistry also underlies cellular communication, such as sensory signal transmission.
Electrochemical Cells
Electrochemical cells is a device that help change chemical energy into electrical energy.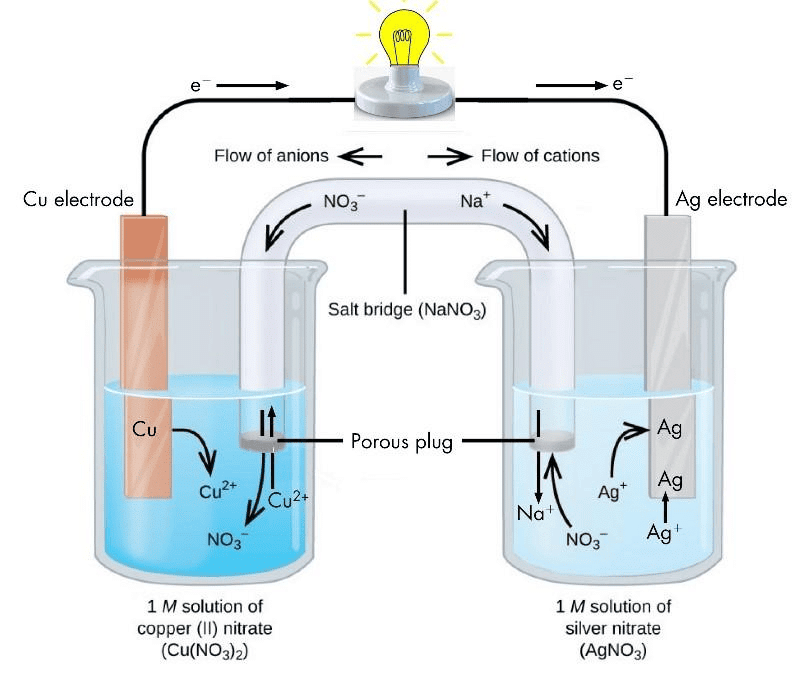
There are two main types of electrochemical cells:
1. Galvanic (Voltaic) Cells: These cells create electric current from spontaneous chemical reactions. A common example is the standard 1.5V battery found in everyday items like remote controls and toys.
2. Electrolytic Cells: These cells require electrical energy to make non-spontaneous chemical reactions happen.
Electrochemical cells have two main parts:
Cathode: The electrode where reduction takes place (it gains electrons).
Anode: The electrode where oxidation occurs (it loses electrons).
Tip to remember: AnOx, RedCat — Anode: Oxidation, Cathode: Reduction
Note:
In a Galvanic (Voltaic) cell, the cathode is positive and the anode is negative. Electrons flow from anode to cathode, and the cell produces electrical energy.
In an Electrolytic cell, the a and the cathode is negative. External electrical energy is used to drive the non-spontaneous reaction.
Half Cells and Cell Potential
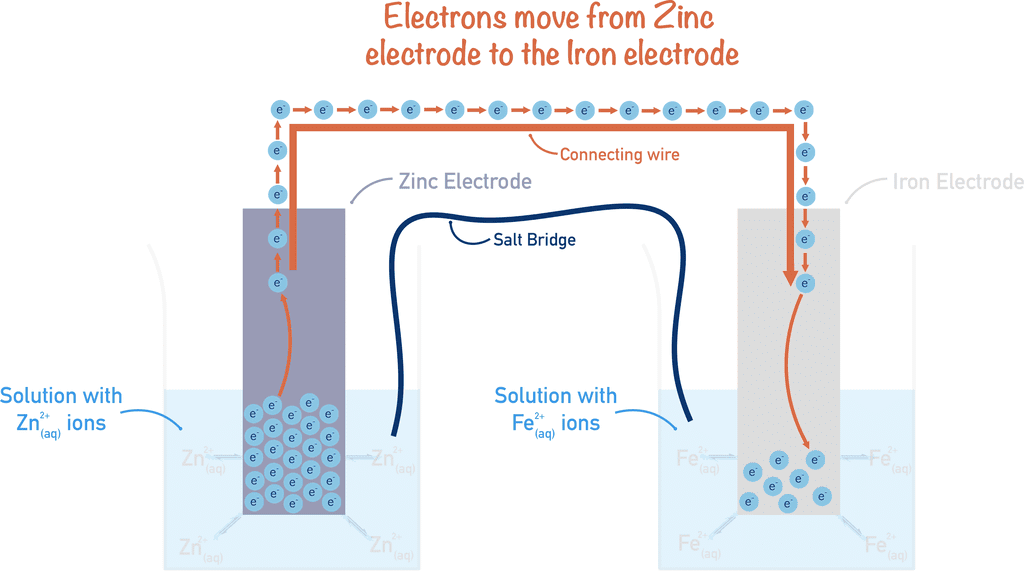
- Electrochemical cells consist of two parts known as half cells.
- Each half cell has an electrode placed in a liquid solution called an electrolyte.
- A Salt bridge links the two half cells, which allows ions to flow between them while keeping the solutions separate.
- The cell potential, or voltage generated by the cell, depends on the electrode potentials of the half cells.
- Electrode potentials are assessed in relation to a standard reference electrode, like the standard hydrogen electrode.
Galvanic Cell
This cell converts chemical energy into electrical energy.
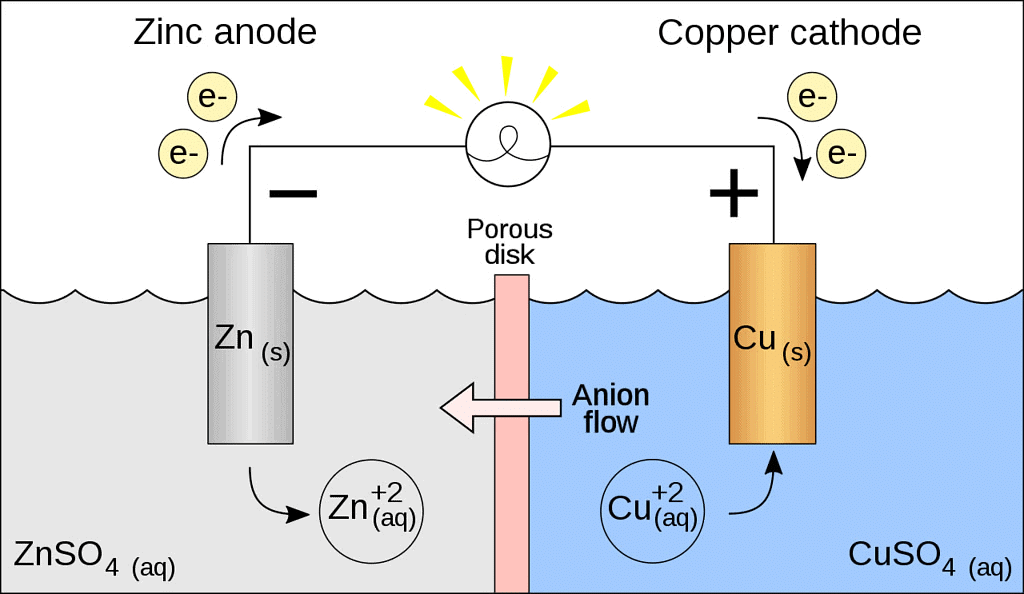 Galvanic Cell
Galvanic Cell
- A galvanic cell is made up of two half cells i.e., anodic and cathodic. The cell reaction is of redox kind. Oxidation takes place at anode and reduction at cathode. It is also known as voltaic cell.
- For Example : Zinc rod immersed in ZnSO4 behaves as anode and copper rod immersed in CuSO4 behaves as cathode.
Oxidation takes place at anode.
Zn → Zn2+ + 2e- (loss of electron : oxidation)
Reduction takes place at cathode:
Cu2- + 2e- → Cu (gain of electron ; reduction)
Overall process: Zn(s) + Cu2+ → Cu(s) + Zn2+
- In galvanic cell like Daniell cell: electrons flow from anode (zinc rod) to the cathode (copper rod) through external circuit; zinc dissolves as Zn2+ ; Cu2+ ion in the cathode cell picks up two electron and become deposited at cathode.
Representation of a cell (IUPAC Conventions):
Let us illustrate the convention taking the example of Galvanic cell.
1. Anodic half cell is written on left and cathodic half cell on right hand side.
Zn(s) |ZnSO4(sol)||CuSO4(sol)|Cu(s)
2. Two half cells are separated by double vertical lines: Double vertical lines indicate slat bridge or any type of porous partition.
3. EMF (electromotive force) may be written on the right hand side of the cell.
4. Single vertical lines indicate the phase separation between electrode and electrolyte solution.
Zn|Zn2+ ||Cu2+ |Cu
5. Invert eletrodes are represented in the bracket
Zn|ZnSO4||H |H2,Pt
Electrolytic Cell
An electrolytic cell can be defined as an electrochemical device that uses electrical energy to facilitate a non-spontaneous redox reaction.
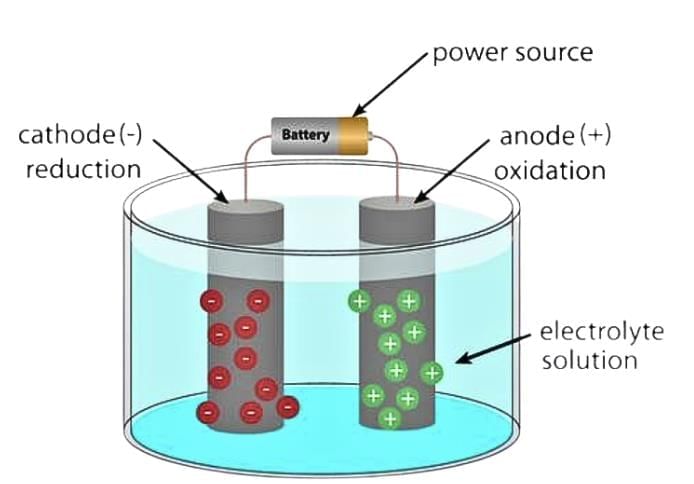
- Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds.
- For example, water can be subjected to electrolysis (with the help of an electrolytic cell) to form gaseous oxygen and gaseous hydrogen.
- This is done by using the flow of electrons (into the reaction environment) to overcome the activation energy barrier of the non-spontaneous redox reaction.
The three primary components of electrolytic cells are:
1. Cathode
(which is negatively charged for electrolytic cells)
2. Anode
(which is positively charged for electrolytic cells)
3. Electrolyte
(The electrolyte provides the medium for the exchange of electrons between the cathode and the anode. Commonly used electrolytes in electrolytic cells include water (containing dissolved ions) and molten sodium chloride.
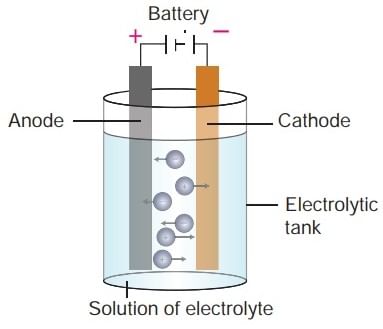 Components of Electrolytic Cell
Components of Electrolytic Cell
values are determined under specific conditions: 1 M concentration, 1 atm pressure for gases, and a temperature of 25°C.
Working of an Electrolytic Cell
An Electrolytic Cell is an electrochemical device that uses an external source of electrical energy to drive a non-spontaneous chemical reaction. It's the opposite of a galvanic or voltaic cell, which spontaneously generates electrical energy from a chemical reaction.
Here's how an electrolytic cell works:
Let's take an example of NaCl
- Molten sodium chloride (NaCl) can be subjected to electrolysis with the help of an electrolytic cell, as illustrated below.
 Working of an Electrolytic Cell
Working of an Electrolytic Cell
- Here, two inert electrodes are dipped into molten sodium chloride (which contains dissociated Na+ cations and Cl– anions).
- The metal strip at which positive current enters is called an anode; the anode is positively charged in an electrolytic cell. On the other hand, the electrode at which the current leaves is called the cathode. Cathodes are negatively charged.

- When an electric current is passed into the circuit, the cathode becomes rich in electrons and develops a negative charge.
- The positively charged sodium cations are now attracted towards the negatively charged cathode.
- This results in the formation of metallic sodium at the cathode. Simultaneously, the chlorine atoms are attracted to the positively charged cathode.
- This results in the formation of chlorine gas (Cl2) at the anode (which is accompanied by the liberation of 2 electrons, finishing the circuit).
- The associated chemical equations and the overall cell reaction are provided below.
Reaction at Cathode: Na+ + e– → Na
Reaction at Anode: 2Cl– → Cl2 + 2e–
Cell Reaction: 2NaCl → 2Na + Cl–
- Thus, molten sodium chloride can be subjected to electrolysis in an electrolytic cell to generate metallic sodium and chlorine gas as the products.
Measurement of Electrode Potential
Concept of electromotive force (EMF) of A Cell
- Electron flows from anode to cathode in external circuit due to a pushing effect called or electromotive force (e.m.f.). EMF is called as cell potential. Unit of e.m.f. of cell is volt.
- EMF of cell may be calculated as:
Ecell = Reduction potential of cathode - Reduction potential of anode
- Similarly, standard e.m.f. of the cell (Eº) may be calculated as
Eºcell = Standard reduction potential of cathode - Standard reduction potential of anode.
Sign Convention of EMF
EMF of cell should be positive other wise it will not be feasible in the given direction.
Zn|ZnSO4||CuSO4|Cu , E = 1.10 volt (Feasible)
Cu|CuSO4||ZnSO4|Zn, E = - 1.10 volt (Not Feasible)
Salt Bridge
Two electrolyte solutions in galvanic cells are separated using salt bridge as represented in the Fig. Salt bridge is a device to minimize or eliminate the liquid junction potential. Saturated solution of salt like KCl, KNO3, NH4Cl and NH4NO3 etc. in agar-agar gel is used in salt bridge.
 Salt bridgeSalt bridge contains high concentration of ions viz. K+ and NO3- at the junction with electrolyte solution. Thus, salt bridge carries whole of the current across the boundary; more over the K+ and NO3- ions have same speed. Hence, salt bridge with uniform and same mobility of cations and anions completes the electrical circuit & permits the ions to migrate.
Salt bridgeSalt bridge contains high concentration of ions viz. K+ and NO3- at the junction with electrolyte solution. Thus, salt bridge carries whole of the current across the boundary; more over the K+ and NO3- ions have same speed. Hence, salt bridge with uniform and same mobility of cations and anions completes the electrical circuit & permits the ions to migrate.
[Nernst Equation]
Nernst Equation
- Electrochemistry is a branch of chemistry that explores the relationship between chemical reactions and electrical energy. A key concept in this field is the Nernst equation, which connects Gibbs free energy with cell potential, allowing for the determination of cell potential and equilibrium constants.
- The Nernst equation accounts for standard electrode potentials, temperature, activity, and the reaction quotient in calculating cell potential. It is derived from the relationship between Gibbs free energy (ΔG\Delta GΔG) and cell potential (EEE), expressed as:
- where n is the number of electrons transferred, and F is Faraday's constant (96,500 C/mol). Under standard conditions, this equation simplifies to:
- In thermodynamics, the Gibbs free energy under non-standard conditions can be related to that under standard conditions and the reaction quotient (Q):
- By substituting the expressions for ΔG and ΔGo into this equation, we arrive at:
- This leads to the Nernst equation:
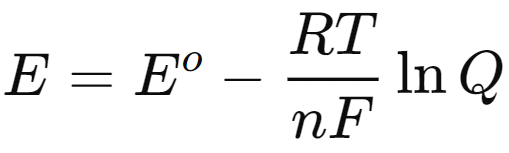
- For practical applications, the equation can be converted to a base-10 logarithm form:
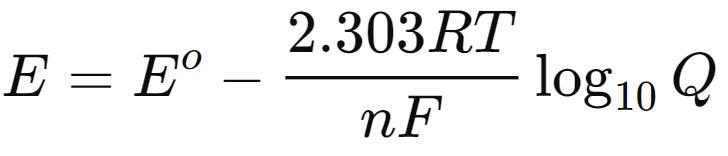
- At standard temperature (298 K), this simplifies to:
This form of the Nernst equation is particularly useful for calculating the potential of electrochemical cells under various conditions, emphasizing the interplay between thermodynamics and electrochemical processes.
Electrochemical cell and Gibbs Energy of the Reaction
Electrical work done in one second is equal to electrical potential multiplied by total charge passed. If we want to obtain maximum work from a galvanic cell then charge has to be passed reversibly. The reversible work done by a galvanic cell is equal to decrease in its Gibbs energy and therefore if the emf of the cell is E and nF is the amount of charge passed and ΔrG is the Gibbs energy of the reaction, then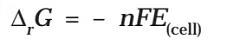 It may be remembered that E(cell) is an intensive parameter but ΔrG is an extensive thermodynamic property and the value depends on n. Thus, if we write the reaction
It may be remembered that E(cell) is an intensive parameter but ΔrG is an extensive thermodynamic property and the value depends on n. Thus, if we write the reaction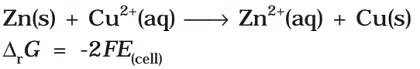 But when we write the reaction
But when we write the reaction
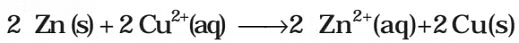
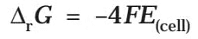 If the concentration of all the reacting species is unity, then E(cell)Thus, from the measurement of E–Cell we can obtain an important thermodynamic quantity, ΔrG–, standard Gibbs energy of the reaction.From the latter we can calculate equilibrium constant by the equation:
If the concentration of all the reacting species is unity, then E(cell)Thus, from the measurement of E–Cell we can obtain an important thermodynamic quantity, ΔrG–, standard Gibbs energy of the reaction.From the latter we can calculate equilibrium constant by the equation: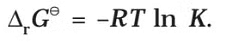
Relationship between ΔG and electrode potential:
- Let n, Faraday charge is taken out from a cell of e.m.f. (E) then electrical work done by the cell may be calculated as, Work done = Charge × Potential = nFE
- From thermodynamics we know that decrease in Gibbs free energy of a system is a measure of reversible or maximum obtainable work by the system if there is no work due to volume expansion ΔG = - nFE
- Under standard state ΔGº = - nFEº ............(1)
1. From thermodynamics we know, DG = negative for spontaneous process. Thus from eq. (i) it is clear that the EMF should be ve for a cell process to be feasible or spontaneous.
2. When ΔG = positive, E = negative and the cell process will be non spontaneous.

- Standard free energy h, range of a cell may be calculated by electrode potential data.
- Substituting the value of Eº (i.e., standard reduction potential of cathode-standard reduction potential of anode) in eq. (1) we may get ΔGº.
What is Electrolysis?
The decomposition of electrolyte solution by passage of electric current, resulting in deposition of metals or liberation of gases at electrodes is known as electrolysis.
Key characteristics of electrolysis include:
- Electrolytic Cell: Electrolysis takes place in an electrolytic cell, which consists of two electrodes (an anode and a cathode) immersed in an electrolyte. The electrodes are usually made of inert materials like platinum or graphite.
- External Electrical Energy: An external power source, such as a battery or direct current (DC) power supply, is connected to the electrodes. This power source provides the necessary electrical potential (voltage) to drive the electrolysis process.
- Redox Reactions: Electrolysis involves redox (reduction-oxidation) reactions. At the anode (positive electrode), oxidation occurs as electrons are removed from the species present, leading to the generation of cations or other products. At the cathode (negative electrode), reduction occurs as electrons are gained by the species present, leading to the formation of anions or other products.
- Ions in the Electrolyte: The electrolyte is a solution or molten compound that contains ions. These ions are involved in the electrochemical reactions at the anode and cathode. For example, in the electrolysis of water, the electrolyte contains H⁺ and OH⁻ ions.
- Migration of Ions: Positive ions (cations) migrate toward the cathode, and negative ions (anions) migrate toward the anode within the electrolyte. This movement of ions helps maintain charge neutrality during the electrolysis process.
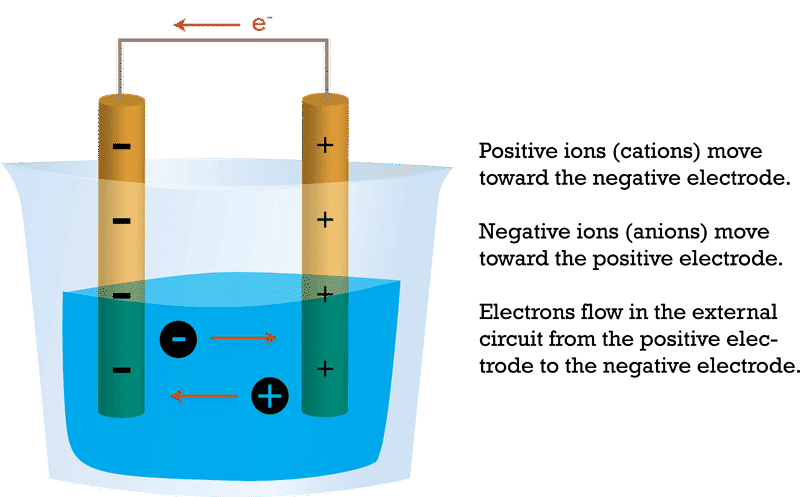 Migration of Ions: Electrolysis
Migration of Ions: Electrolysis - Product Formation: The chemical reactions at the anode and cathode result in the production of new substances. These substances can be collected or used for various purposes. For example, in the electrolysis of water, hydrogen gas is produced at the cathode, and oxygen gas is produced at the anode.
Faraday's Laws of Electrolysis
These laws describe the relationship between the amount of substance deposited or produced during an electrolysis process and the quantity of electricity (electric charge) passed through the system.
- Faraday's Laws of Electrolysis are two fundamental principles in electrochemistry established by the English scientist Michael Faraday in the early 19th century.
- Faraday's Laws laid the foundation for the quantitative understanding of electrolysis and electrochemical reactions. There are two Faraday's Laws of Electrolysis:
1. First law of electrolysis:
The amount of substance deposited or liberated at an electrode is directly proportional to the amount of charge passed (utilized) through the solution.
| W ∝ Q W = ZQ |
where
- W = weight liberated
- Q = charge in coulomb
- Z = electrochemical equivalent
when Q = 1 coulomb, then W = Z
Thus, weight deposited by a 1-coulomb charge is called electrochemical equivalent.
Let 1-ampere current is passed till 't' seconds.
Then, Q = It => W = ZIt
| W = ZIt |
1 Faraday = 96500 coulomb = Charge of one mole electrons
One faraday is the charge required to liberate or deposit one gm equivalent of a substance at the corresponding electrode.
Let 'E' is equivalent weight then 'E' gm will be liberated by 96500 coulombs.
1 Coulomb will liberate gm
By definition, Z =
W =
When gas is evolved at an electrode, then the above formula changes as,
V =
where V = volume of liberated gas, Ve = equivalent volume of gas.
Equivalent volume may be defined as:
The volume of gas liberated by 96500 coulombs at STP.
Hence, According to Faraday's first law of electrolysis: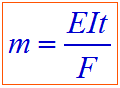
Where:
- m = mass of substance
- E = equivalent weight of substance
- I = current in amperes
- t = time required
- F = 96,500 Coulombs
2. Second law of electrolysis:
When the same amount of charge is passed through different electrolyte solutions connected in series then the weight of substances deposited or dissolved at anode or cathode is in the ratio of their equivalent weights. i.e.
| w1/w2 = E1/E2 |
Solved Examples on Faraday's Laws of Electrolysis
Q.1. Electrolysis of dilute aqueous NaCl solution was carried out by passing 10 milliampere current. The time required to liberate 0.01 mol of H2 gas at the cathode is:
a) 9.65 x 104 s
b) 28.95 x 104 s
c) 19.3 x 104 s
d) 38.6 x 104 s
Solution:
Mass of H2 = m = 0.01 mol x 2 g mol-1
Equivalent weight of H2 = E = Atomic weight / valence = 1 g mol-1 / 1 = 1 g mol-1
Current in ampere = I = 10 milliamperes = 10 x 10-3 amperes.
Conclusion: The correct option is 'c'.
Q.2. A 4.0 molar aqueous solution of NaCl is prepared and 500 mL of this solution is electrolyzed. This leads to the evolution of chlorine gas at one of electrodes (relative atomic mass of Na = 23, Hg = 200; 1 Faraday constant = 96500 Coulombs mol-1) :
The total number of moles of chlorine gas evolved is:
a) 3.0
b) 2.0
c) 0.5
d) 1.0
Solution:
First we have to calculate the amount of NaCl present in 4.0 molar solution.
The number of moles of NaCl in 500 mL of 4.0 molar solution = Molarity x Volume (in L) = 4.0 x 500 x 10-3 = 2.0 moles.
The following reaction occurs during electrolysis of aqueous solution of NaCl.Dissociation of NaCl: 2 NaCl(aq) ------> 2Na+(aq) + 2Cl-(aq)
At cathode: 2H2O + 2e- -------> H2 + 2OH-
At anode: 2Cl-(aq) ---------> Cl2(g) + 2e-
Complete reaction: 2NaCl(aq) + 2H2O ------>H2 + Cl2(g) + 2Na+(aq) + 2OH-(aq)
That means, two moles of NaCl gives one mole of Cl2 gas.
Since there are 2.0 moles of NaCl present in the solution, one mole of Cl2 gas will be evolved at anode upon complete electrolysis.
Conclusion: The correct option is 'd'.
Products of Electrolysis
1. Products of electrolysis depend on the material being electrolyzed. In other words, the nature of electrolyte governs the process of electrolysis. The process is fast for a strong electrolyte whereas for a weak electrolyte an extra potential better known as overpotential is required.
Products of electrolysis depend on upon the value of this over potential too.
2. Products of electrolysis depend on the nature of electrodes too. That is, in the case of the inert electrode (say gold, platinum), it doesn’t participate in the reaction whereas if the electrode used is reactive in nature it takes part in the reaction.
3. Various oxidising and reducing species present in the electrolytic cell do affect the products of electrolysis.
4. The products of electrolysis depend on standard electrode potentials of the different oxidizing and reducing species present in the electrolytic cell.
5. In the case of multiple reactions, the product of electrolysis depends on the standard electrode potential of various reactions taking place. For example, electrolysis of an aqueous solution of sodium chloride. Out of the multiple reduction reactions taking place, the reduction reaction which has the highest value of standard electrode potential takes place at the cathode. Similarly, out of the multiple oxidation reactions, the oxidation reaction which has the lowest value of standard electrode potential takes place at the anode.
The SRP Values at 25º C for some of the reduction half-reaction are given in the table below.
S. NO. | Reduction half cell reaction | Eo in volts at 250 |
1. | F2 + 2e- →→ 2F- | + 2.65 |
2. | S2O82- + 2e- → 2SO42- | + 2.01 |
3. | Co3+ + e- → Co2+ | + 1.82 |
4. | PbO2 + 4H+ + SO42- + 2e- → PbSO4 + 2H2O | + 1.65 |
5. | MnO4- + 8H+ + 5e- → Mn2+ + 4H2O | + 1.52 |
6. | Au3+ + 3e- → Au | + 1.50 |
7. | Cl2 + 2e- → 2Cl- | + 1.36 |
8. | Cr2O2-7 + 14 H+ + 6e- → 2Cr3+ + 7H2O | + 1.33 |
9. | O2 + 4H+ + 4e- → 2H2O | + 1.229 |
10. | Br2 + 2e- → 2Br- | + 1.07 |
11. | NO3- + 4H+ + 3e- → NO + 2H2O | + 0.96 |
12. | 2Hg2+ + 2e- → Hg22+ | + 0.92 |
13. | Cu2+ + I- + e- → CuI | + 0.86 |
14. | Ag+ + e- → Ag | + 0.799 |
15. | Hg22+ + 2e- → 2Hg | + 0.79 |
16. | Fe3+ + e- → Fe2+ | + 0.77 |
17. | I2 + 2e- → 2I- | + 0.535 |
18. | Cu+ + e- → Cu | + 0.53 |
19. | Cu2+ + 2e- → Cu | + 0.34 |
20. | Hg2Cl2 + 2e- → 2Hg + 2Cl- | + 0.27 |
21. | AgCl + e- → Ag + Cl- | + 0.222 |
22. | Cu2+ + e- → Cu+ | + 0.15 |
23. | Sn4+ + 2e- → Sn2+ | + 0.13 |
24. | 2H+ + 2e- → H2 | + 0.00 |
25. | Fe3+ + 3e- → Fe | - 0.036 |
26. | Pb2+ + 2e- → Pb | - 0.126 |
27. | Sn2+ + 2e- → Sn | - 0.14 |
28. | Agl + e- → Ag + I- | - 0.151 |
29. | Ni2+ + 2e- → Ni | - 0.25 |
30. | Co2+ + 2e- → Co | - 0.28 |
31. | Cd2+ + 2e- → Cd | - 0.403 |
32. | Cr3+ + e- → Cr2+ | - 0.41 |
33. | Fe2+ + 2e- → Fe | - 0.44 |
34. | Cr3+ + 3e- → Cr | - 0.74 |
35. | Zn2+ + 2e- → Zn | - 0.762 |
36. | 2H2O + 2e- → H2 + 2OH- | - 0.828 |
37. | Mn2+ + 2e- → Mn | - 1.18 |
38. | Al3+ + 3e- → Al | - 1.66 |
39. | H2 + 2e- → 2H- | - 2.25 |
40. | Mg2+ + 2e- → Mg | - 2.37 |
41. | Na+ + e- → Na | - 2.71 |
42. | Ca2+ + e- → Ca | - 2.87 |
43. | Ba2+ + 2e- → Ba | - 2.90 |
44. | Cs+ + e- → Cs | - 2.92 |
45. | K+ + e- → K | - 2.93 |
46. | Li+ + e- → Li | - 3.03 |
- When a solution of an electrolyte contains more than one type of cations and anions at concentrations different than 1 M, the discharge of an ion does not depend solely on standard potentials but also depends on the concentration of an ion in the solution.
- The value is referred to as potential, called reduction potential for cation and oxidation potential for the anion. The relation between reduction potential and standard reduction potential is given by the Nernst Equation, as
- Where ERP = Reduction potential of cation and EºRP = Standard reduction potential of the cation.
- Thus, it is possible that a cation (A+ ) with lower standard reduction potential getting discharged in preference to cation (B+) having higher standard reduction potential because their concentration might be such that the reduction potential of A is higher than that of B+.
- When two metal ions in the solution have identical values of their reduction potentials, the simultaneous deposition of both the metals will occur in the form of an alloy.
Practice Questions
Q.1. What is the product formed at the cathode in the electrolysis of molten NaCl?
a) Chlorine gas
b) Sodium metal
c) Hydrogen gas
d) Oxygen gas
Answer: b
Explanation: In the electrolysis of NaCl, if the electrolyte is molten NaCl, then the only ions formed after dissociation are Na+ and Cl– ions. The cathode being a negatively charged electrode attracts the positive Na+ ions and neutralizes it to form Sodium metal.
Q.2. What is the product formed at the cathode in the electrolysis of aqueous Na2SO4?
a) Sodium metal
b) Oxygen gas
c) Hydrogen gas
d) Sulphur
Answer: c
Explanation: Na2SO4 dissociates into Na+ and SO42- ions in the electrolysis of aqueous Na2SO4. Na+ has much lower reduction potential than water and hence Na+ ions are not reduced at the cathode. Instead, reduction of water occurs giving out hydrogen gas at the cathode.
Q.3. What is the product formed at the cathode in the electrolysis of aqueous CuSO4?
a) Copper metal
b) Oxygen gas
c) Hydrogen gas
d) Sulphur
Answer: a
Explanation: In the electrolysis of aqueous CuSO4, Cu2+, SO42+, H+ and OH– are the ions formed after dissociation. Copper ions have much higher reduction potential than water. Hence, these ions are easily reduced and deposited as Cu at the cathode.
Q.4. What are the two electrodes used in Daniell cell?
a) Pt and Cu
b) Al and Zn
c) Al and Pt
d) Zn and Cu
Answer: d
Explanation: The two electrodes that are used in a Daniell cell are zinc (as anode) and copper (as cathode) electrodes which are dipped in a solution containing its own ions, generally zinc sulphate and copper sulphate.
Q.5. What is the electrolyte used in the electroplating of gold?
a) Molten gold
b) [AgCN2]–
c) AuCN
d) AuCl3
Answer: c
Explanation: The electrolyte in electrolysis should contain the metal to be coated, gold in this case. AuCN is used because it is exceptionally stable and doesn’t resist the flow of Au+ ions from anode to cathode.
|
75 videos|278 docs|78 tests
|
FAQs on Electrolytic Cells, Electrolysis & Products of Electrolysis - Chemistry Class 12 - NEET
| 1. What is electrochemistry and why is it important? |  |
| 2. What are electrochemical cells and how do they function? |  |
| 3. What is the difference between a galvanic cell and an electrolytic cell? |  |
| 4. How does the Nernst equation relate to electrochemical cells? |  |
| 5. What is electrolysis and what are its applications? |  |