Emulsions | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Emulsions |

|
| Function of Emulsifier |

|
| Properties of Emulsion |

|
| Demulsification |

|
| Use of Emulsions |

|
| Gels |

|
Emulsions
An emulsion is a colloidal solution of a liquid. It may be defined as a heterogeneous system consisting of more than one immiscible liquids dispersed in one another in the form of droplets whose diameter, in general, exceeds 0.1 m.
For example, milk is an emulsion in which small drops of liquid fat are dispersed in aqueous medium. Cod liver oil is an emulsion in which the water drops are dispersed in the oil. This means in most of the emulsions one of the liquid is water and the other liquid is oil. Here the term 'oil' is used to represent all organic substances which are soluble in water.
Types of Emulsions
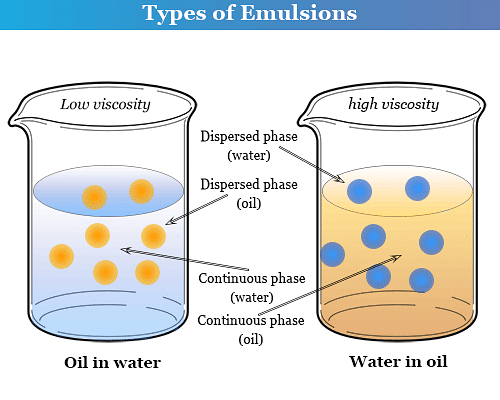
(1) Oil in water type emulsion (O/W): In this emulsion, oil is the dispersed phase and water is the dispersion medium. It is denoted by O/W or O in W. For example, milk (liquid fat dispersed in water), vanishing cream, etc.
(2) Water in oil type : In this emulsion, water is the dispersed phase and oil is the dispersion medium. It is denoted by W/O or W in O. For example, butter, cod liver oil, cold cream, etc.
The type of emulsion obtained by agitating two immiscible liquids depends upon the relative amounts of two components liquids. The liquid that is in excess forms the dispersion medium. Thus, the two types of emulsions can be inter-converted into each other by changing the concentration of one of the liquids.
The distinction between two types of Emulsions
The two types of emulsions may be distinguished from each other in a number of ways.
(1) Dye test : It involves the addition of oil soluble dye to the emulsion under experiment. If the emulsion acquires the colour of the dye readily, it is water-in-oil type emulsion and it the emulsion remains colourless, it is oil-in-water type emulsion.
(2) Conductivity test : It involves the addition of electrolyte to the emulsion under experiment. If the conductivity of the emulsion increases appreciably with the addition of electrolyte, it is
oil-in-water type emulsion and it conductivity is very small, it is water-in-oil type emulsion.
(3) Dilution test : As a general rule, an emulsion can be diluted with the dispersion medium while the addition of the dispersed phase forms a separate layer. Thus, if an emulsion can be diluted with oil, it is a water-in-oil type.
Preparation of emulsion (Emulsification)

Emulsification is the process which involves the preparation of emulsion. Generally, an emulsion is prepared by subjecting a mixture of the immiscible liquid to a distinct layers upon standing. The oil globules rise to form an upper layer while aqueous medium forms lower layers. To prevent the separation of layers and to get the stable emulsion, a small quantity of the third substance is added. This substance that stabilizes the emulsion is called emulsifier or emulsifying agent. The commonly used emulsifying agents are soaps, detergents and lyophilic colloids. Casein, a lyophilic colloid present in milk, acts as an emulsifier as it forms a protective layer around fat molecules dispersed in water. Hence milk is a fairly stable emulsion.
Function of Emulsifier
The main function of emulsifier or emulsifying agents is to lower the interfacial tension between oil and water and thus helps the intermixing of two liquids. For example, a molecule of a soap or detergent (emulsifier) gets concentrated at the interface between oil and water. The polar end of the emulsifier is in water and non-polar end is in oil as shown in figure.
In a soap, RCOONa, R is the non-polar end, whereas COO— Na is the polar end.
Properties of Emulsion
(i) The size of particles of the dispersed phase of an emulsion is usually larger than in sols.
(ii) Like colloidal particles, emulsions exhibit properties such as Tyndall effect, Brownian movement (provided the particles are not too large), electrophoresis, coagulation, etc.
Demulsification
The process which involves the breaking of an emulsion into two separate liquid layers is called demulsification. The following methods may be used to bring demulsification:
(1) Chemical Methods : An emulsion may be demulsified by adding a chemical substance whose action on the dispersed phase and the dispersion medium is opposite to that of the original emulsifying agent used to produce the stable emulsion.
(2) Centrifugation : Cream is separated from milk by the centrifugal method.
(3) Cooling : Fat can be removed from milk by keeping it in a refrigerator for a few hours.
Demulsification :
Besides the above noted methods of demulsification, the following methods have also been developed:
(i) Suitable centrifugal action-milk cream is separated from milk by centrifugation.
(ii) Application of electric field-electrophoresis.
(iii) Addition of an electrolyte having multivalent opposite charge than that on the dispersed phase.
(iv) Chemical destruction of stabiliser.
(v) Distilling off of one of the components, usually water.
(vi) Addition of demulsifiers like alcohol, phenol etc.
Use of Emulsions
(1) Many pharmaceutical preparations-medicines, ointments, creams and various lotions are emulsions. It is believed that medicines are more effective and easily assimilated by the body tissues when they are in colloidal form i.e., emulsion.
(2) All paints are emulsions.
(3) The digestion of fat in the intestines is helped by emulsification. A little of the fat forms a medium soap (emulsifier) with the alkaline solution of the intestine and this soap emulsifier the rest of the fats, thus making it easier for the digestive enzymes to do their metabolic functions.
(4) Soaps and detergents remove dust and dirt from the dirty piece of cloth by making an oil in water emulsion.
(5) Milk is an emulsion of liquid fats in water.
(6) In the process of metallurgy, one of the important steps is the concentration of ore which is usually done by froth floatation process in which an oil is added to the finely-divided ore taken in water. The particles of ore go on the surface due to formation of foams while the other impurities are left at the bottom of the vessel.
(7) The emulsion of asphalt in water is used in road making and building.
Gels
Colloidal system in which liquids are the dispersed phase and solid act as the dispersion medium are called gels. The common examples are : boot polishes, jelly, gum arabic, agar agar, processed cheese and silicic acid.
When the gels are allowed to stand for a long time, they give out small quantities of trapped liquids with accumulate on its surface. This action of gels is known as Synresis or Weeping. Some gels such as silica, gelatin and ferric hydroxide liquify on shaking and reset on allowing to stand. This phenomenon of Sol-gel transformation is called thixotropy.
Gels are divided into two categories i.e. elastic gels and non elastic gels. The two categories differ from their behaviour towards dehydration and rehydration as under.
| Elastic gels | Non-elastic gels |
| 1. They change to solid mass on dehydration which can be changed back to original form by addition of water followed by warming. | 1. They change to solid mass on dehydration which cannot be changed back to original form with water. |
| 2. They absorb water when placed in it with simultaneous swelling. This phenomenon is | 2. They do not exhibit imbibation called imbibation. |
|
108 videos|286 docs|123 tests
|
FAQs on Emulsions - Chemistry Class 12 - NEET
| 1. What is the function of an emulsifier? |  |
| 2. What are the properties of emulsions? |  |
| 3. What is demulsification? |  |
| 4. What are some common uses of emulsions? |  |
| 5. What are some examples of colloids around us? |  |

|
Explore Courses for NEET exam
|

|

















