Enzymes & Nucleic Acids | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| Enzyme |

|
| Mechanism of Enzyme-Catalyzed Reactions |

|
| Nutrients |

|
| Vitamins |

|
| Nucleic Acids |

|
| Deoxyribonucleic Acid (DNA) |

|
| Ribonucleic Acid (RNA) |

|
| Types of RNA and their Functions |

|
Enzyme
Enzymes are biological catalysts produced by living cells that speed up biochemical reactions essential for life.

Nature of Enzymes
- Mostly globular proteins, though some non-protein enzymes exist.
- They are highly specific and selective in their actions, unlike other catalysts.
Importance
- Without enzymes, life-sustaining processes would occur far too slowly.
- Example: In the absence of digestive enzymes, it would take around 50 years to digest a single meal.
Classification and Naming
- Typically named after the compound or type of reaction they catalyze.
- Example:
1. Maltase: Breaks down maltose into glucose.
2. Oxide Reductase: Facilitates oxidation-reduction reactions.
Function
- Enzymes lower the activation energy of reactions, significantly increasing reaction rates.
Statistics
- Approximately 3,000 enzymes have been identified.
- Only around 10% (or 300) are commercially available for use.
Some common Enzymes and the reactions which are catalysed by them are given in the Table below:
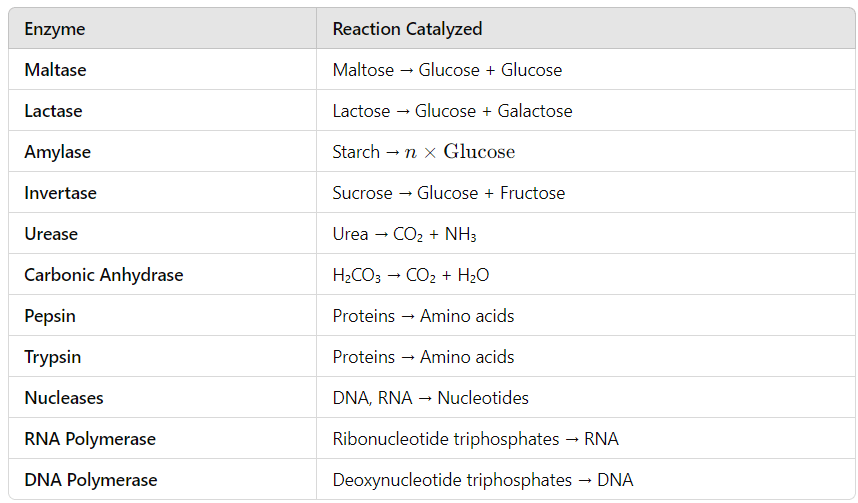
Properties :
1. High Efficiency
- Enzymes can speed up reactions by up to 10 million times compared to uncatalyzed reactions.
- This efficiency is due to enzymes lowering the activation energy required for reactions.
- Example: The activation energy for acid hydrolysis of sucrose is 6.22 kJ mol⁻¹, while with the enzyme sucrase, it is only 2.15 kJ mol⁻¹.
2. Extremely Small Quantities
- Enzymes are effective even in minuscule amounts (as small as a millionth of a mole).
- Small enzyme quantities can increase reaction rates by factors ranging from 10³ to 10⁶.
3. Specificity
- Enzymes are highly specific to particular reactions.
- Each biochemical reaction has a specific enzyme that catalyzes it.
- Example: Maltase specifically catalyzes the hydrolysis of maltose.
4. Optimum Temperature and pH
- Enzymes work best at moderate conditions, around 37°C and pH 7.
5. Control of Activity
- Enzyme activity is regulated through various mechanisms.
- Enzymes can be inhibited by different organic and inorganic molecules.
5.Regulation of Activity
- Most enzymes have tightly controlled activity, ensuring precise regulation of biochemical reactions.
Mechanism of Enzyme-Catalyzed Reactions
Biochemists study the molecular process of enzyme-catalyzed reactions, which involve the following steps:
1. Formation of Enzyme-Substrate Complex:
- The enzyme (E) binds with the substrate (S) to form an enzyme-substrate complex, represented as E + S → ES.
- This complex is called the enzyme-substrate complex (ES).
2. Product Formation:
- Within the complex, the substrate is converted into a product, forming an enzyme-product complex (EP).
- This step is represented as ES → EP.
3. Product Release:
- The product (P) is released, and the enzyme reverts to its original state.
- This step is represented as EP → E + P.
These steps are depicted in the Figure , showing the enzyme binding with the substrate, forming the product, and then releasing it.
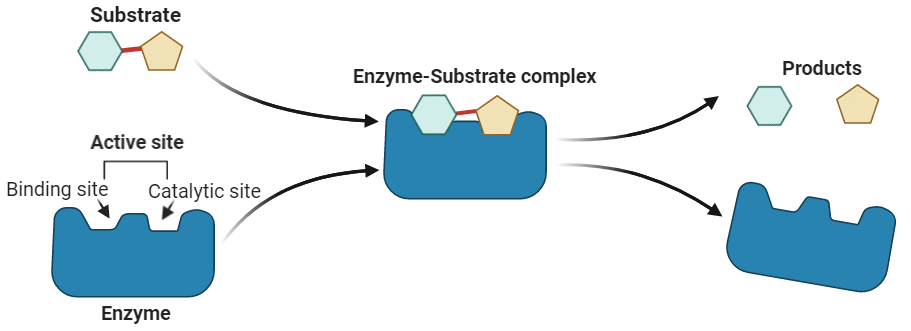
Enzymes have specific regions on their surface called active sites or catalytic sites, where the reaction takes place. These active sites have a unique shape that specifically fits the substrate molecules. This "fit" between the enzyme’s active site and the substrate is highly selective, similar to a key fitting into a lock. This precise fit ensures that only particular substrates can bind to each enzyme, allowing for specific biochemical reactions.
Nutrients
These are the chemical substances that are required for the proper functioning of cells, tissues, and organs in living organisms. Nutrients are essential for growth, repair, and protection against disease-causing microbes.

Some of the important Nutrients are:
Sodium, Potassium and Chlorine
- Na+ is the principal mineral cation in the extracellular fluid.
- K + is the principal cation inside the cell.
- Cl- is the principal mineral anion in the ECF.
- Na+ and K+ are essential to the maintenance of water balance and acid-base balance.
- Na+ and K+ are important in nerve impulse transmission.
Calcium and Phosphorus
- Calcium and phosphorus are deposited in bones and teeth to give them strength and rigidity.
- Ca2+ is also essential for blood coagulation, neuromuscular function, cardiac function and actions of many enzymes and hormones.
- Phosphorus enters into many compounds such as nucleic acids and phospholipids, many co-enzymes and high energy compounds like ATP.
- Calcium plays an essential role in sustaining intestinal peristalsis and growth of body tissues.
Iron
- Iron is required for haemoglobin synthesis.
- Iron is essential both for transportation of oxygen to tissues and for operation of oxidative systems within the tissue cells.
Magnesium
- Magnesium is required as a catalyst for many intracellular enzymatic reactions, particularly those relating to carbohydrate metabolism.
- Mg is the central metal atom in chlorophyll.
Iodine
- Iodine is used in the synthesis of thyroid hormones.
Zinc
- Zinc is a constituent of carbonic anhydrase, present in RBCs helping in CO2 transport.
- Zinc is a component to lactic dehydrogenase, important for the interconversion between pyruvic acid and lactic acid.
- Zinc is a component part of some peptidases and therefore is important for digestion of proteins in the alimentary canal.
Cobalt
- Cobalt helps in erythropoiesis and in the activities of some enzymes.
- It is present in vitamin B12 .
Copper
- Copper helps in the utilisation of iron.
- Copper deficiency may produce anaemia because of failure in iron utilisation.
Molybdenum
- Molybdenum is a constituent of oxidase enzymes (xanthine oxidase).
- Molybdenum plays an important role in biological nitrogen fixation.
Fluorine
- Fluorine maintains normal dental enamel and prevents dental caries.
- Excessive intake of fluorine cause fluorosis characterized by mottled teeth and enlarged bones.
Vitamins
It has been observed that certain organic compounds are required in small amounts in our diet but their deficiency causes specific diseases. These compounds are called vitamins.
Classification of Vitamins
Vitamins are classified into two groups depending upon their solubility in water or fat.
1. Fat soluble vitamins:
Vitamins which are soluble in fat and oils. But insoluble in water are kept in this group. These are vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues.
2. Water soluble vitamins:
B group vitamins and vitamin C are soluble in water so they are grouped together. Water soluble vitamins must be supplied regularly in diet because they are readily excreted in urine and can not be stored (except vitamin B12) in our body.
Some important vitamins, their sources and diseases caused by their deficiency are listed in table.

Nucleic Acids
Nucleic acids are polynucleotides, composed of repeating units called nucleotides. These are special type of acids which are present in nucleus & cytoplasm. It control the metabolic activities of cell. They are also found in Mitochondria, centriole and chloroplast.
There are two types of nucleic acids:
- DNA (Deoxyribonucleic Acid)
- RNA (Ribonucleic Acid)
Each nucleotide consists of:
1. A nitrogenous base
2. A five-carbon sugar
3. A phosphate group
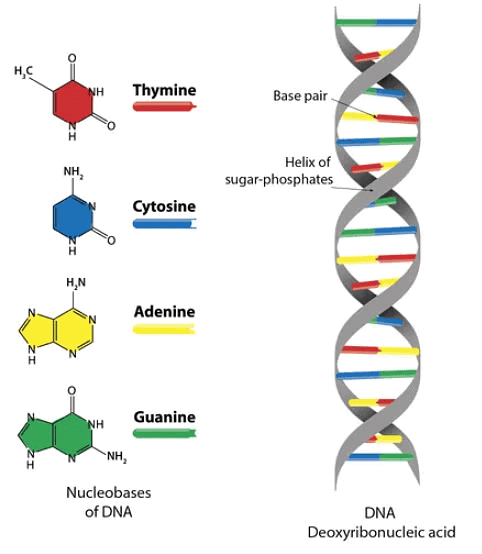
1. Nitrogen containing Heterocyclic Base
The nitrogenous bases fall into two types:
- Purines (two rings): adenine (A) and guanine (G)
- Pyrimidines (one ring): cytosine (C), thymine (T), and uracil (U)
The Structure of these compounds are shown below:
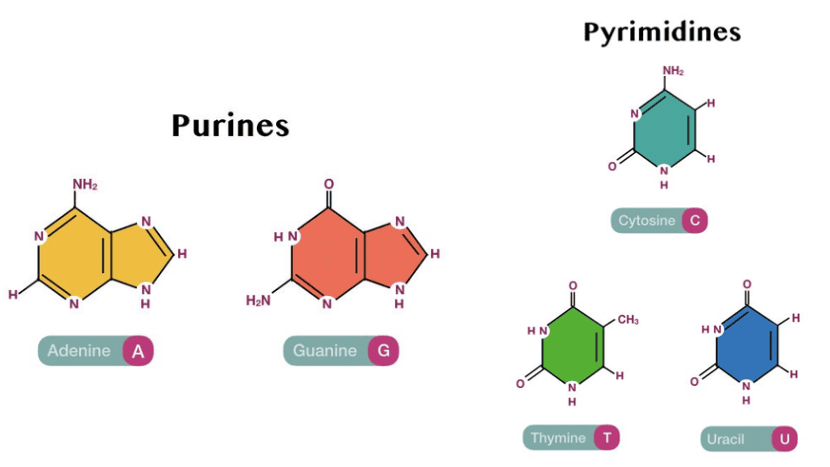
2. Sugars
There are two types of sugars present in nucleic acids. The sugars present in RNA is ß-D-ribose and in DNA is ß-D-2 deoxyribose as shown below :
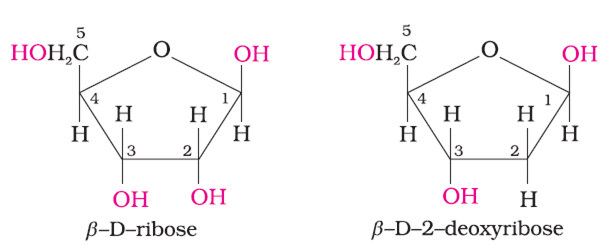
3. A Phosphate group
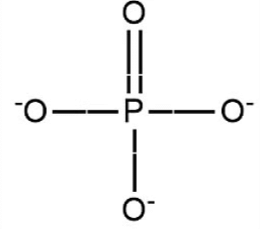
These are responsible for the linkage in nucleic acids.
The phosphate group in nucleic acid is:
Deoxyribonucleic Acid (DNA)
It is found in Nucleus. They are on pneumococcus bacteria.
- DNA made up of 3 units-
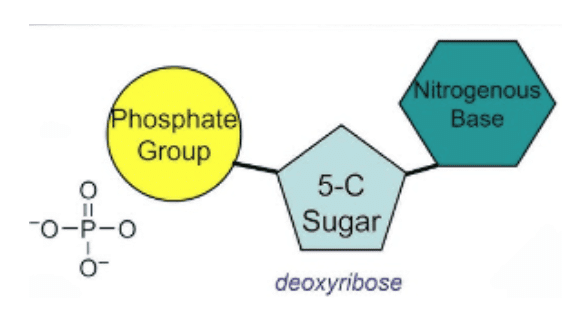
- Nucleoside - When nitrogen base is combined with deoxyribose sugar, it constitutes a nucleoside.
Deoxyribonucleoside:
Adenine Deoxyribose → Deoxyadenosine
Guanine Deoxyribose → Deoxyguanosine
Cytosine Deoxyribose → Deoxycytidine
Thymine Deoxyribose → Deoxythymidine - Nucleotide
- Nitrogen base Sugar Phosphate → Nucleotide.
- Nucleotide is a unit of DNA.
- All nucleotides combined and form a chain called polynucleotides by which RNA and DNA are formed.
Structure of DNA
- Double Helical model of DNA was proposed by biochemist J.D.Watson, British chemist FHC Crick in 1953.
- DNA in double stranded structure is made up of two chains of polynucleotides.
- DNA is a polymer of Nucleotide.
- Nucleotide are joined by 3' → 5' phosphodiester bonds.
- Sugar and phosphorous are alternately arranged.
- In both chains, in between A and T, 2 Hydrogen bonds are present while in C and G, 3H bonds are present. (A = T) (C º G)
- A always attaches with T while C always attaches with G.
- Purine and pyrimidine are found in ratio 1 : 1 cells.
- DNA is attached with histone protein.
- In prokaryotic cell and mitochondria, circular DNA is present.
Functions of DNA
1. Self - Replication or self -Duplication
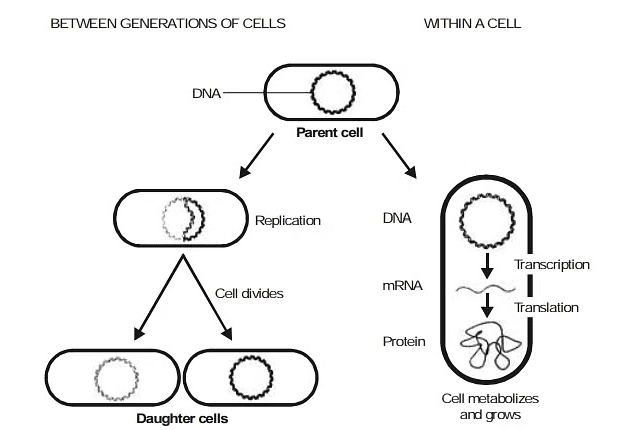
DNA has a property of self - replication. It is therefore a reproducing molecule. This unique property of DNA is at the root of all reproduction. Through its replication, DNA acts as the key to heredity. In the replication of DNA, the two strands of a double helix unwind and separate as a template for the formation of a new complementary strand.
2. Protein Synthesis
The specific sequence of base pair in DNA represents coded information for the manufacture of specific proteins. These coded instructions first are transcribed into the matching nitrogen-base sequences within mRNA and the instructions in such RNA subsequently are translated into particular sequence of amino acid units within the polypeptide chains and proteins.
The major steps in the utilization of the genetic information can be represented as :
Ribonucleic Acid (RNA)
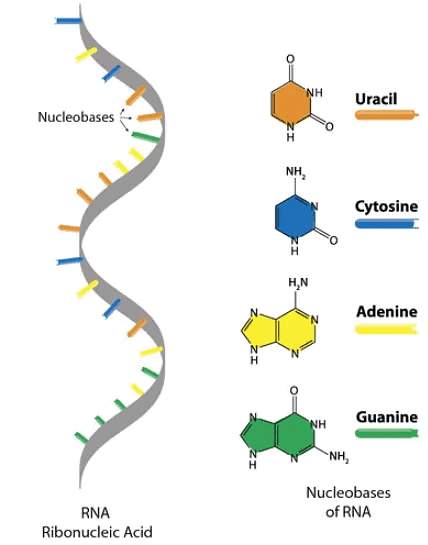
It is a molecule that is present in all living cells and most viruses. It is a nucleic acid, like DNA, that is made up of nucleotides, which are ribose sugars attached to phosphate groups and nitrogenous bases. It is found in cytoplasm as well as in nucleus.
 Chemical Nature
Chemical Nature
- Ribonucleic acid is a polymer of purine and pyrimidine ribonucleotides linked by 3' → 5' phosphodiester bridges. The number of nucleotides in RNA ranges from as few as 75 to many thousands. Although sharing many features with DNA, RNA possesses several specific differences.
- As indicated by its name, sugar in RNA to which the phosphate and nitrogen- bases are attached is ribose rather than the deoxyribose of DNA.
- Although RNA contains the ribonucleotides of adenine, guanine, and cytosine, it does not posses thymine. Instead of thymine, RNA contains the ribonucleotides of uracil. Thus, the pyrimidine components of RNA differs from those of DNA.
- RNA exists basically as a single-stranded molecule rather than as a double -stranded helical molecule, as does DNA. However the single strand of RNA is capable of folding back on itself like a hairpin and thus acquiring double-stranded characteristics. In these regions, A pairs with U and G pairs with C.
- Thus a given segment of a long RNA molecule might, for example, be represented as follows:
- where R stands for ribose ; A, U, G, and C for Adenine, Uracil, Guanine and Cytosine respectively.
Types of RNA and their Functions
There are 3 main types of RNA molecules:
1. Messenger RNA (mRNA)
2. Transfer RNA (tRNA)
3. Ribosomal RNA (rRNA)
1. Messenger RNA (mRNA)
- This type of RNA consists of single strand of variable length and serves as a template for protein synthesis. Code is in the chromosomes.
- mRNA forms complementary copy of DNA as it carries chemical messages in the form of nitrogen-base sequence from the nucleus to the ribosomes, i.e. from DNA to cytoplasm where proteins are synthesized. Therefore, it is called messenger RNA or mRNA.
- mRNA is sythesised from DNA in the nucleus.
- It is called transcription.
2. Ribosomal RNA
- A ribosome is a cytoplasmic nucleoprotein structure which serves as the organellar machinery for protein synthesis from mRNA templates.
- On the ribosome, the mRNA and tRNA molecules interact to translate into a specific protein molecule the information transcribed from the DNA.
- rRNA constitutes the largest part of total RNA (Highest) - 80%
3. Transfer RNA (RNA)
- These are also called Soluble RNA.
- Single stranded.
- 10-15% of the total RNA.
- Size - Smallest: 75 - 80 nucleotides only.
- Synthesis - Within nucleus from DNA.
Function- It transports amino acid from cytoplasm to the site of protein synthesis.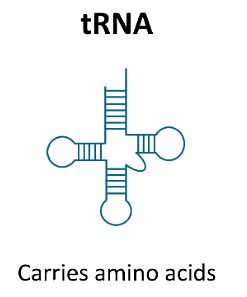
|
75 videos|278 docs|78 tests
|
FAQs on Enzymes & Nucleic Acids - Chemistry Class 12 - NEET
| 1. What are the main factors that affect enzyme activity? |  |
| 2. How do vitamins function as coenzymes in enzymatic reactions? |  |
| 3. What is the chemical composition of nucleic acids? |  |
| 4. What are the main functions of DNA in living organisms? |  |
| 5. What are the different types of RNA and their functions? |  |




















