Ionic Equilibrium, Solubility Product & Common Ion Effect | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| Introduction to Ionic Equilibrium |

|
| Ostwald’s Dilution Law: Degree of Dissociation |

|
| Common Ion Effect on Degree of Dissociation |

|
| Solubility Equilibra of Spraingly Soluble Salts |

|
Ionic Equilibrium - Ostwald Dilution law
In Ionic equilibrium, the ionic substance dissociates into their ions in polar solvents. The ions formed are always in equilibrium with its undissociated solute in the solution.
⇒ Representation of Ionic Equilibrium: Xa Yb ⇌ aXb+ + bYa-
Introduction to Ionic Equilibrium
Reactants and products coexist in equilibrium so that reactant conversion to product is always less than 100%. Equilibrium reactions may involve the decomposition of a covalent (non-polar) reactant or ionization of ionic compounds into their ions in polar solvents.
In this section, we will learn about the ionic equilibrium in ionic solutions. Substances in Ionic Equilibrium can be classified into two categories on the basis of their ability to conduct electricity given as under,
Non-Electrolytes
These are substances that consist of molecules that bear no electric charge, do not dissociate into their constituent ions and thus do not conduct electricity in their aqueous solution or molten state. For example sugar solution.
Electrolytes
These are substances that dissociate into their constituent ions in their aqueous solution and thus conduct electricity in their aqueous solutions or molten state. Example, salt solution, acid solution, base solution etc.
Electrolytes in ionic equilibrium can be further classified into strong and weak electrolytes.
Strong electrolytes are substances that upon dissociation in their ionic solution ionize completely while in the case of weak electrolytes, the dissociation is partial in nature.
For example, NaCl undergoes complete ionization in its aqueous solution to render sodium ions (Na+) and chloride (Cl–) ions, whereas, acetic acid undergoes partial ionization to render some amount of acetate ions(CH3COO–) and hydrogen(H+) ions.
- In case of a strong electrolyte, the dissociation reaction is said to be complete, thus moving in the forward direction only, whereas, in case of a weak electrolyte, the reaction is said to be reversible in nature.
- In the case of the weak electrolyte, the equilibrium is established between the ions and the unionized molecules, which can be termed as ionic equilibrium. The same can be understood with the following example.
Ostwald’s Dilution Law: Degree of Dissociation
Ostwald’s dilution law is the application of the law of mass action to weak electrolytes in solution.
A binary electrolyte AB which dissociates into A+ and B– ions.
AB ⇌ A+ + B–
(i) For very weak electrolytes, since α <<< 1, (1 – α) = 1
∴ K = Cα2 α = √KV
(ii) Concentration of any ion = Cα = √CK = √K/V
Degree of ionization increases on dilution. Thus, degree of dissociation of a weak electrolyte is proportional to the square root of dilution.
Limitations of Ostwald’s Dilution law
The law holds good only for weak electrolytes and fails completely in the case of strong electrolytes.
Ionic Equilibrium Formulas
It becomes necessary to know what fraction of the initial amount of the reactants are converted into products at equilibrium.
The fraction of the initial molecules that are converted at equilibrium is called the degree of Dissociation / ionization.
Degree of dissociation or ionization = α = (Number of reactant molecules dissociated\ionized at the start) / (Number of reactant molecules at the start)
Degree of dissociation in Ionic equilibrium can be expressed in percentage.
% Degree of dissociation or ionization = α = (Number of reactant molecules dissociated or ionized at the start)/(Number of reactant molecules at the start) × 100
Degree of Ionization
The degree of ionization depends on
- Nature of the electrolyte: strong, weak, insoluble
- Nature of the solvent: High dielectric solvents increase ionization
- Dilution: larger the dilution higher the ionization
- Temperature: higher the temperature, larger the ionization and
- Presence of common ions decreases the ionization of the weak electrolyte.
Dissociation of Ionic Compounds in Polar Solvents
Ionic compounds dissolve in polar solvents with ionization into cations and anions.
The ionized ions are in equilibrium with the un-dissociated molecules.
AxBy ⇌ xAy+ + yBx-
Ionic Solids in Solutions
Strong electrolytes (α ≈100% ionization), Weak electrolytes (α ≈ 10% ionization), Sparingly soluble (α ≈100% ionization)
Example: HCl, NaOH, Salts NH4OH, Organic acids AgCl, BaSO4
Ionization of Weak Electrolytes
In infinite dilution, all electrolytes are fully ionized. In a concentrated solution, weak electrolytes exist in equilibrium with their unionized molecules. Concentrations of the ions are important in many practical situations like acid-base solubility, and conductance of the solution.
Common Ion Effect on Degree of Dissociation
Weak electrolytes are poorly ionized in aqueous solution. Their ionization may further be reduced if one of the ions are present from another source. This called a common ion effect.
- Ammonium hydroxide is a weak base. On addition of ammonium chloride (a salt) ammonium ion from it, will make the ammonium ions to combine with the hydroxide to form unionized ammonium hydroxide.
NH4Cl → NH4+ + Cl–
NH4OH ⇌ NH4+ + OH– - In the base hydrolysis of oil, the sodium salt of the fatty acid (soap) is in a dissolved state. When sodium chloride salt is added, the concentration of Na+ ions increases considerably.
CnH2n+1 + COONa ⇌ CnH2n+1 COO– + Na+
NaCl ⇌ Na+ + Cl–
Hence, the ionic product [CnH2n+1COO–] [Na+] exceeds the solubility product of soap and, therefore, soap precipitates out from the solution. This is called salting out of soap. - Manufacture of sodium bicarbonate (baking soda): In Solvay’s soda process. The CO2 gas is passed through ammonical brine to precipitate out NaHCO3.
NH4OH + CO2 → NH4HCO3
NH4HCO3 + NaCl → NH4HCO3 + NH4Cl
NaHCO3 is precipitated first because of its lower solubility product as compared to those of NH4Cl, NH3HCO3 and NaCl.
Solubility Equilibra of Spraingly Soluble Salts
Solubility Product (Ksp)
The solubility product constant is the equilibrium constant for the dissolution of a solid substance into an aqueous solution. It is denoted by the symbol Ksp.
The solubility product is a kind of equilibrium constant and its value depends on temperature. Ksp usually increases with an increase in temperature due to increased solubility.
Solubility is defined as a property of a substance called solute to get dissolved in a solvent in order to form a solution. The solubility of ionic compounds (which disassociate to form cations and anions) in water varies to a great deal. Some compounds are highly soluble and may even absorb moisture from the atmosphere whereas others are highly insoluble.
Significance of Solubility Product
Solubility depends on a number of parameters amongst which lattice enthalpy of salt and solvation enthalpy of ions in the solution are of most importance.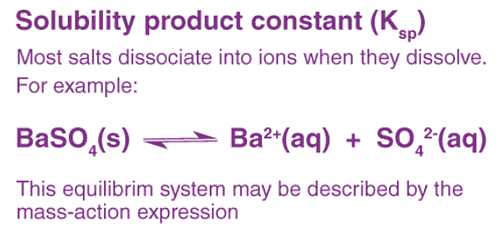
- When a salt is dissolved in a solvent the strong forces of attraction of solute (lattice enthalpy of its ions) must be overcome by the interactions between ions and the solvent.
- The solvation enthalpy of ions is always negative which means that energy is released during this process.
- The nature of the solvent determines the amount of energy released during solvation that is solvation enthalpy.
- Non-polar solvents have a small value of solvation enthalpy, meaning that this energy is not sufficient to overcome the lattice enthalpy.
- So the salts are not dissolved in non-polar solvents. Hence, for salt to be dissolved in a solvent, its solvation enthalpy should be greater than its lattice enthalpy.
- Solubility depends on temperature and it is different for every salt.
Salts are classified on the basis of their solubility in the following table: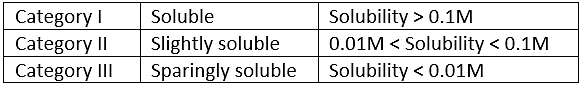
Solubility Product Constant
Suppose barium sulphate along with its saturated aqueous solution is taken.
The following equation represents the equilibrium set up between the undissolved solids and ions:
The equilibrium constant in the above case is: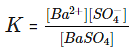
In case of pure solid substances the concentration remains constant, and so we can say:
Here Ksp is known as the solubility product constant. This further tells us that solid barium sulphate when in equilibrium with its saturated solution, the product of concentrations of ions of both barium and sulphate is equal to the solubility product constant.
Factors affect the value of Ksp
Some important factors that have an impact on the solubility product constant are:
- The common-ion effect (the presence of a common ion lowers the value of Ksp).
- The diverse-ion effect (if the ions of the solutes are uncommon, the value of Ksp will be high).
- The presence of ion-pairs.
Common Ion Effect
The common ion effect is an effect that suppresses the ionization of an electrolyte when another electrolyte (which contains an ion which is also present in the first electrolyte, i.e. a common ion) is added. It is considered to be a consequence of Le Chatlier’s principle (or the Equilibrium Law).
What is the Common Ion Effect?
The statement of the common ion effect can be written as follows – in a solution wherein there are several species associating with each other via a chemical equilibrium process, an increase in the concentration of one of the ions dissociated in the solution by the addition of another species containing the same ion will lead to an increase in the degree of association of ions.
An example of the common ion effect can be observed when gaseous hydrogen chloride is passed through a sodium chloride solution, leading to the precipitation of the NaCl due to the excess of chloride ions in the solution (brought on by the dissociation of HCl).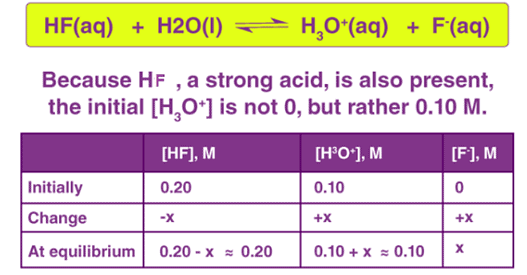 This effect cannot be observed in the compounds of transition metals. This is because the d-block elements have a tendency to form complex ions. This can be observed in the compound cuprous chloride, which is insoluble in water. This compound can be dissolved in water by the addition of chloride ions leading to the formation of the CuCl2– complex ion, which is soluble in water.
This effect cannot be observed in the compounds of transition metals. This is because the d-block elements have a tendency to form complex ions. This can be observed in the compound cuprous chloride, which is insoluble in water. This compound can be dissolved in water by the addition of chloride ions leading to the formation of the CuCl2– complex ion, which is soluble in water.
Effect on Solubility
The way in which the solubility of a salt in a solution is affected by the addition of a common ion is discussed in this subsection.
- The common ion effect can be used to obtain drinking water from aquifers (underground layer of water mixed with permeable rocks or other unconsolidated materials) containing chalk or limestone. Sodium carbonate (chemical formula Na2CO3) is added to the water in order to decrease the hardness of the water.
- In the treatment of water, the common ion effect is used to precipitate out the calcium carbonate (which is sparingly soluble) from the water via the addition of sodium carbonate, which is highly soluble.
- A finely divided calcium carbonate precipitate of a very pure composition is obtained from this addition of sodium carbonate. The CaCO3 precipitate is, therefore, a valuable by-product which can be used in the process of manufacturing toothpaste.
- Since soaps are the sodium salts of carboxylic acids containing a long aliphatic chain (fatty acids), the common ion effect can be observed in the salting-out process which is used in the manufacturing of soaps. The soaps are precipitated out by adding sodium chloride to the soap solution in order to reduce its solubility.
However, it can be noted that water containing a respectable amount of Na+ ions, such as seawater and brackish water, can hinder the action of soaps by reducing their solubility and therefore their effectiveness.
pH and the Common-Ion Effect
When the conjugate ion of a buffer solution (solution containing a base and its conjugate acid, or acid and its conjugate base) is added to it, the pH of the buffer solution changes due to the common ion effect.
- An example of such an effect can be observed when acetic acid and sodium acetate are both dissolved in a given solution, generating acetate ions. However, sodium acetate completely dissociates but the acetic acid only partly ionizes. This is because acetic acid is a weak acid whereas sodium acetate is a strong electrolyte.
- As per Le Chatelier’s principle, the new acetate ions put forth by sodium acetate facilitate the suppression of the ionization of acetic acid, thereby shifting the equilibrium to the left. Since the dissociation of acetic acid is reduced, the pH of the solution is increased.
- Therefore, the common ion solution containing acetic acid and sodium acetate will have an increased pH and will, therefore, be less acidic when compared to an acetic acid solution.
Thus, the common ion effect, its effect on the solubility of a salt in a solution, and its effect on the pH of a solution.
|
127 videos|245 docs|87 tests
|
FAQs on Ionic Equilibrium, Solubility Product & Common Ion Effect - Chemistry Class 11 - NEET
| 1. What is ionic equilibrium in solution? |  |
| 2. How does ionic equilibrium affect pH? |  |
| 3. What factors can disrupt ionic equilibrium in a solution? |  |
| 4. How can we calculate the degree of dissociation in an ionic equilibrium? |  |
| 5. What are some common examples of ionic equilibrium in everyday life? |  |



















