Group 17 Elements: Halogens Family | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| What are Halogens? |

|
| General Physical Properties of Group 17 Elements: |

|
| Chemical Properties of Group 17 Elements: |

|
| Interhalogen Compounds |

|
| Compounds of Group 17 Elements |

|
What are Halogens?
The halogens are the elements that form group 17 of the periodic table. They are reactive nonmetals and include fluorine, chlorine, bromine, and iodine.
Halogens are highly reactive non-metals. These elements greatly resemble in property with each other. Group 17 elements are collectively called halogens (In Greek: halo means salt and genes mean producing, so collectively salt-producing) and it consists of fluorine, chlorine, bromine, iodine, and astatine.
 Group 17 - Halogens
Group 17 - Halogens
The similarity to this extent is not found in other groups of the periodic table. They have a regular gradation in the physical and chemical properties. Astatine is the only radioactive element in the group. They have seven electrons in their outermost shell (ns2np5) and are short of one electron from the configuration of the nearest noble gas. The chemical properties and reactivity of an element are determined by the oxidation state exhibited by them.
General Physical Properties of Group 17 Elements:
Electronic configuration:
Their valence shell electronic configuration is ns2 np5.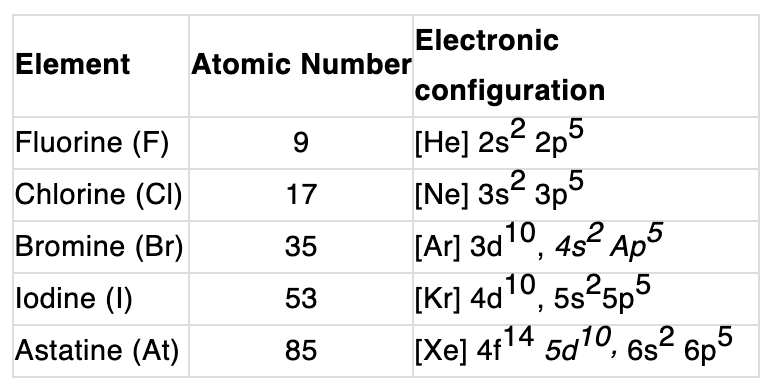
Physical state:
Intermolecular forces in halogens are weak and increase down the group. Thus, F2 and Cl2 are gases, Br2 is volatile liquid and I2 is solid.Atomicity:
All are diatomic in nature.Abundance:
Being very reactive in nature, they are not found free in nature. Their presence in the earth’s crust follows the order: F2 > Cl2 > Br2 > I2Colour:
They absorb light in the visible range forming excited states and are thus, coloured in nature.
F2 ⇒ pale Yellow
Cl2 ⇒ yellowish green
Br2 ⇒ reddish brown
I2 ⇒ deep violetMetallic character:
All the elements are non-metals and metallic character increases down the group. Thus, 1 forms 1+.Oxidation state:
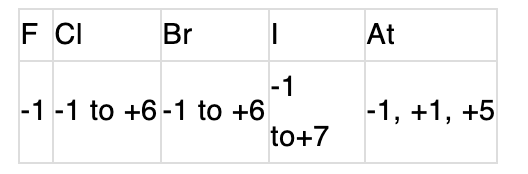
Bond energy & bond length:
The bond length increases from fluorine to iodine and in the same order bond energy decreases. However, the bond dissociation energy of F2 is lesser due to its smaller size. The order of bond energy is: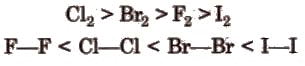
Density:
It increases down the group in a regular fashion and follows the order F > Cl > Br >I.Ionisation enthalpy:
The ionisation enthalpy of halogens is very high and decreases down the group. The iodine also forms I+ and I3+ and forms compounds like LiI, lCN, IPO4. In a molten state, the compounds conduct electricity showing ionic character.Electron affinity:
The halogens have high values for electron affinity. The order of electron affinity is: Cl2 > F2 > Br2 > I2
Due to the small size of fluorine (hence, high electron density), the extra electron to be added feels more electron-electron repulsion. Therefore, fluorine has less value for electron affinity than chlorine.Reduction potentials and oxidising nature:
E°red of halogens are positive and decrease from F to I. Therefore, halogens act as strong oxidising agents and their oxidising power decreases from fluorine to iodine. Fluorine is the strongest oxidising agent and is most reactive. That’s why it is prepared by the electrolysis of a mixture of KHF2 and anhydrous HF using Monel metal as a catalyst.Solubility:
Halogens are soluble in water which follows the order: F2 > Cl2 > Br2 > I2
The solubility of iodine in water is enhanced in the presence of KI.
KI + I2 ⇔ KI3 ⇔ K+ + I–2
I2 forms a blue colour complex with starch.
Chemical Properties of Group 17 Elements:
Hydrides:
HF is a low boiling liquid due to intermolecular hydrogen bonding, while HCI, HBr, HI are gases. The boiling point follows the trend: HF > Hi > HBr > HCl
Some other properties show the following trend: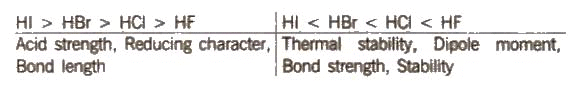
Oxides:
Fluorine forms two oxides, OF2 and O2F2, but only OF2 is thermally stable at 298K. O2F2 oxidises Plutonium to PuF6 and the reaction is used for removing plutonium as PuF6 from spent nuclear fuel.
Chlorine forms a number of oxides such as, CI2O, CI2O3, Cl2O5 , Cl2O7 , CIO2 and CIO2 is used as a bleaching agent for paper pulp, textiles and in water treatment.
Br2O, BrO2, BrO3 are the least stable bromine oxides and exist only at low temperatures. They are very powerful oxidising agents.
The iodine oxides, i.e., I2O4, I2O5, I2O7 are insoluble solids and decompose on heating. I2O5 is a very good oxidising agent and is used in the estimation of carbon monoxide.Reaction with alkali:
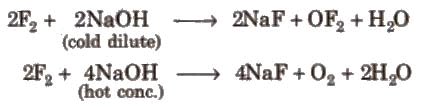 Other halogens form hypohalite with dilute NaOH and hypohalite with conc. NaOH4.
Other halogens form hypohalite with dilute NaOH and hypohalite with conc. NaOH4.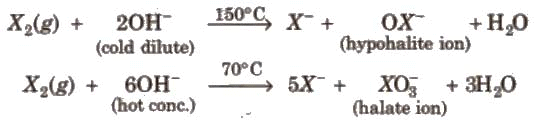
Oxoacids of halogens:
Higher oxoacids of fluorine such as HFO2, HFO3 do not exist because fluorine is the most electronegative and has the absence of d-orbitals.
+3 oxidation state of bromine and iodine are unstable due to the inert pair effect. Therefore, HBrO2 and HIO2 do not exist.
Acidic character of oxoacids decreases as the electronegativity of the halogen atom decreases. Thus, the order of acidic strength. For the oxoacids of the same halogens. acidic strength and thermal stability increase as the number of O atoms increases.
For the oxoacids of the same halogens. acidic strength and thermal stability increase as the number of O atoms increases.
Interhalogen Compounds
When two different halogens react with each other, interhalogen compounds are formed. These compounds are covalent and diamagnetic in nature. They are volatile solids or liquids except for elf which is a gas at 298 K. Interhalogen compounds are more reactive than halogens (except fluorine).
- The XY3 type compounds have bent ‘T’ shape, XY5 type compounds have square pyramidal shape and IF7 has pentagonal bipyramidal structure.
- BrF3 has “T” shaped structure due to 3 bp and 2 lp.
- ICI is more reactive than I2 due to a weak bond. ClF3 and BrF3 are used for the production of UF6 in the enrichment of 235 U.
- U(s) + 3CIF3(l) → UF6(g) + 3CIF(g)
Pseudohalogens and Pseudohalides
The substances behaving like halogens are known as pseudohalides. Some examples are
Pseudohalogen | Pseudohalide ion |
(CM)2 Cyanogen | CN- Cyanide |
(OCN)2 Oxycyanogen | OCN- Cyanate |
(SCN)2 Thiocyanogen | SCN- Thiocyanate |
Compounds of Group 17 Elements
1. Chlorine(i) Occurrence:
Common salt, NaCl is most important. Chlorine is also present in sea water and as rock salt.
(ii) Preparation of Chlorine:
- By oxidation of conc. HCl:
4NaCl + Mn02 + 4H2SO4 → 4NaHSO4 + MnCl2 + 2H2O + Cl2 - Weldon’s process:
MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 - Deacon’s process:
In this process, HCl is oxidised by O2 in the presence of CuCl2 as a catalyst at 400oC.
4HCl + O2 → 2Cl2 + 2H2O - Electrolytic process: By the electrolysis of brine solution in Nelson cell.
2Na+ + 2e- → 2Na + H2O → 2NaOH + H2 (at cathode)
2Cl- → 2Cl + 2e- → Cl2 (at anode)
(iii) Properties:
It is yellowish-green gas, collected by upward displacement of air poisonous in nature, soluble in water. Its aqueous solution is known as chlorine water.
(iv) Chemical Reactions:
- Action of water:
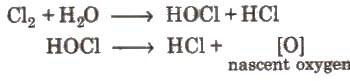 Coloured matter + [0] → colourless matter. The bleaching action of chlorine is due to oxidation and is permanent.
Coloured matter + [0] → colourless matter. The bleaching action of chlorine is due to oxidation and is permanent. - Action of hydrogen:
- Displacement reactions:
- Action of NaOH (cold):
An aqueous solution of NaOCl is called Javelle water,
- Action of H2S:
- Action of dry SO2:
- Action of CO:
- Oxidising properties:
- Reaction with ammonia:
- Chromyl chloride test: When a mixture of chloride and solid K2Cr2O7 is heated with concentrated H2SO4 in a dry test tube, deep red vapours of chromyl chloride are evolved.
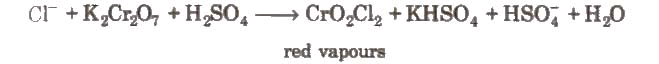 When these vapours are passed through NaOH solution, the solution becomes yellow due to the formation of sodium chromate.
When these vapours are passed through NaOH solution, the solution becomes yellow due to the formation of sodium chromate.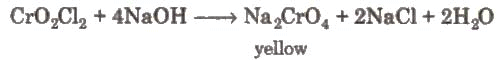 The yellow solution is neutralised with acetic acid and on the addition of lead acetate gives a yellow precipitate of lead chromate.
The yellow solution is neutralised with acetic acid and on the addition of lead acetate gives a yellow precipitate of lead chromate.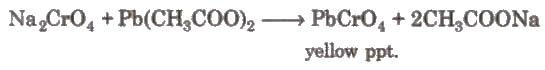
(v) Uses:
It is used as a bleaching agent, disinfectant and in the manufacture of CHCl3, CCl4, DDT, anti-knocking compounds and bleaching powder.2. Hydrochloric Acid (HCl)
(i) Preparation:
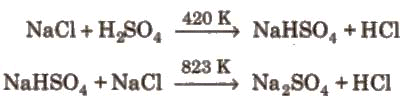
(ii) Properties:
It is a colourless and pungent-smelling gas. It is extremely soluble in water and ionises as below: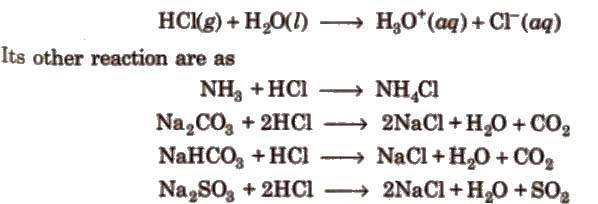
[Noble metals like gold, platinum can dissolve in aqua-regia [three part conc. HCl and one part of conc. HNO3].
(iii) Uses:
It is used in the manufacture of chlorides. Chlorine, in textile and dyeing industries, in medicine and in the extraction of glue from animal tissues and bones.
3. Iodine (I2):
- Its major source is deep seaweeds of laminaria variety. Their ashes which are called kelp contain 0.5% iodine as iodides.
- Another source of I2 is caliche or crude chile saltpetre (NaNO3) which contains 0.2%, NaIO3. Iodine is purified by sublimation.
- It shows no reaction with water. The tincture of iodine is a mixture of I2 and KI dissolved in rectified spirit.
4.Oxoacids of Halogens:
Due to high electronegativity and small size, fluorine forms only one oxoacid, HOF known as fluoric (I) acid or hypofluorous acid. The other halogens form several oxoacids. Most of them cannot be isolated in pure state. They are stable only in aqueous solutions or in the form of their salts.
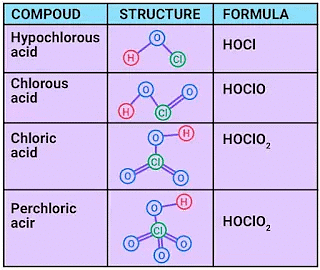 Oxoacids of Chlorine
Oxoacids of Chlorine
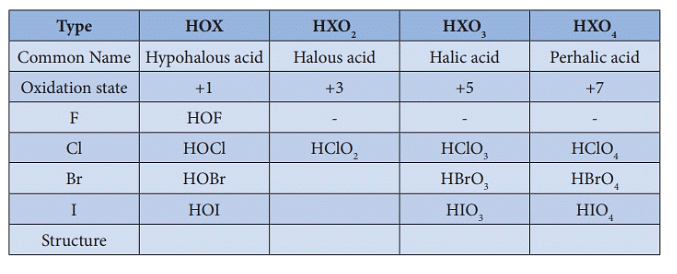 Oxoacids of Halogens
Oxoacids of Halogens
|
75 videos|278 docs|78 tests
|
FAQs on Group 17 Elements: Halogens Family - Chemistry Class 12 - NEET
| 1. What are halogens? |  |
| 2. What are the general physical properties of Group 17 elements? |  |
| 3. What are the chemical properties of Group 17 elements? |  |
| 4. What are interhalogen compounds? |  |
| 5. What are chlorine and its compounds? |  |
















