Organometallic Chemistry: Hapticity of Ligands & 18 Electron Rule | Inorganic Chemistry PDF Download
| Table of contents |

|
| Organometallic Compounds |

|
| Concept of Hapticity in Ligands |

|
| The 18 Electron Rule |

|
| Electron Counting Methods |

|
| Applications of Organometallic Compounds |

|
Organometallic Compounds
An organometallic compound is generally defined as one that possesses a metal – carbon bond.
- Organometallic chemistry can be viewed as bridge between organic and inorganic chemistry.
- For example, all chemists would undoubtedly characterize nickel Tetracarbonyl, Ni(CO)4, as an Organometallic compound even though carbon monoxide is hardly a typical organic compound.
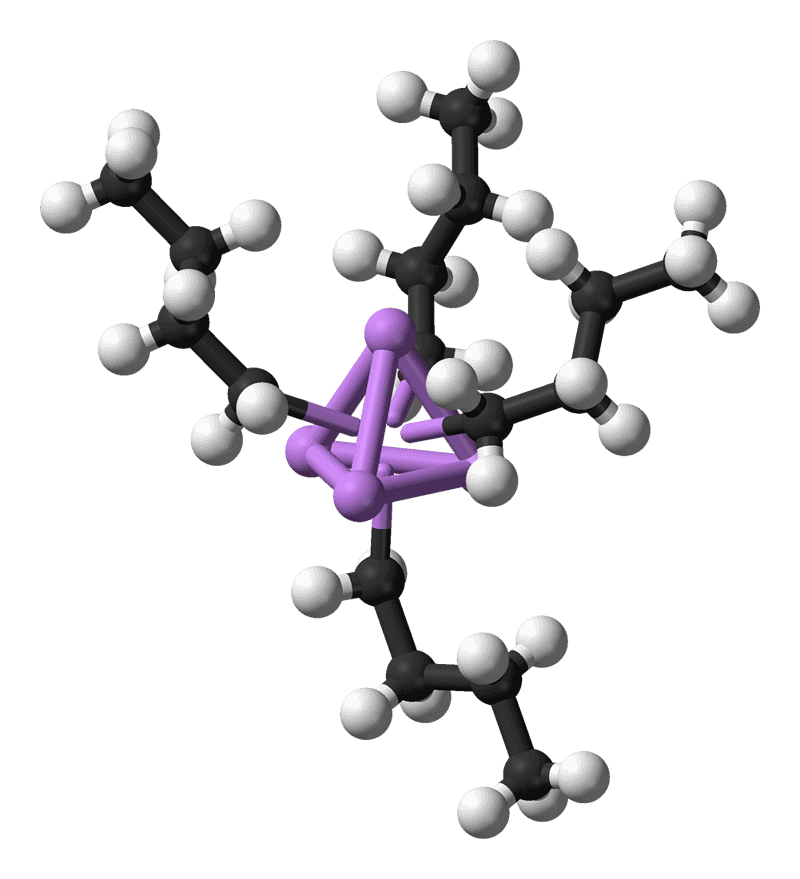 n-Butyllithium, an organometallic compound. Four lithium atoms (in purple) form a tetrahedron, with four butyl groups attached to the faces (carbon is black, hydrogen is white).
n-Butyllithium, an organometallic compound. Four lithium atoms (in purple) form a tetrahedron, with four butyl groups attached to the faces (carbon is black, hydrogen is white).
- Likewise organoboron, organosilicon, organoarsenic, and organotellurium compounds are included in organometallic chemistry even though boron, silicon, arsenic, and tellurium are borderline metals.
- Traditional inorganic chemicals such as sodium cyanide, although possessing a metal-carbon bond, are not normally categorized as organometallic compounds.
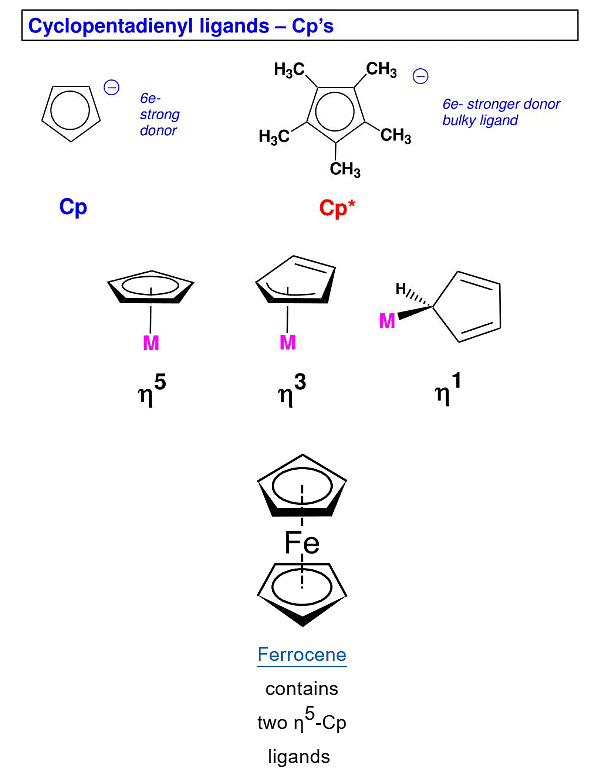
Concept of Hapticity in Ligands
Hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter η ('eta').
- A single organic ligand may interact with a central metal atom using one or more of its atoms simultaneously.
- The number of atoms in a ligand attached to the metal atom is denoted by the prefix η followed by a superscript indicating the number of ligand atoms attached to the metal atom. This called hapticity.
- Most ligands attach through one atom only, therefore, they are called as monohapto (η1).
- Cyclopentadienyl ligand, C5H5- or Cp, for example, can attach to metal atom through one, three or five carbon atoms. Therefore, it may act as mono (η1), tri(η3)- or penta hapto (η5)-ligand.
The 18 Electron Rule
Atoms tend to have all its valence orbitals occupied by paired electrons. For transition metals, the valence orbitals consist of ns, 3 np and 5 (n-1)d orbitals, leading to its tendency of being surrounded by 18 electrons
- The 18 Electron Rule is a useful tool to predict the structure and reactivity of organometallic complexes.
- It describes the tendency of the central metal to achieve the noble gas configuration in its valence shell and is somewhat analogous to the octet rule in a simplified rationale.
- Exceptions to this rule exist, depending on the energy and character of atomic and molecular orbitals.
- This is somewhat analogous to the octet and Lewis structure rules of main group elements in a simplified rationale.
- Structures that satisfy this preferred electron structure are described as electron-precise. Transition metal complexes with 18 electrons are also referred to as saturated, and there will be no other empty low-lying orbitals available for extra ligand coordination.
- Complexes with less than 18 electron counts are unsaturated and can electronically bind to additional ligands.
Exceptions to The Rule
- The 18 electron rule is usually followed in metal complexes with strong field ligands that are good σ donors and π acceptors (for example, CO ligands). The energy difference (Δ0) between t2g and eg* orbitals is very large, and in this case the three t2g orbitals become bonding and are always filled, while the two eg* orbitals are strongly antibonding and are always empty.
- However, when Δ0 between t2g and eg* orbitals are small, for example, in the case of first row transition metals with weaker field ligands, the antibonding character of eg* orbitals weakens, and the complex can have up to 22 electrons.
- On the other hand, less than 18 electrons may be observed in complexes of 4th and 5th row transition metals with high oxidation states. In this case, Δ0 is relatively large due to increased repulsion between d orbitals of metals and the ligands. The eg* orbitals are strongly antibonding and remains empty, while t2g orbitals are non-bonding, and may be occupied by 0-6 electrons.
- Still, generally, the types of ligands in a complex determine if the complex would follow the 18 electron rule or not.
A few common examples of exceptions to 18 electron rules include:
- 16-electron complexes: The metal center is usually low-spin and is in d8 configuration. These complexes adopt square planar structure, such as Rh(I), Ni(II), Pd(II), and Pt(II) complexes. In a lot of catalytic reactions, the organometallic catalysts convert back and forth between 18 and 16 electron configurations, and thus completes a catalytic cycle.
- Bulky ligands may hinder the completion of 18 electron rule. Examples include complexes with agostic interaction.
- Complexes with ligands of strong π-donating characters often violate 18 electron rule. Examples of this kind of ligands include F-, O2-, RO- and RN2-.
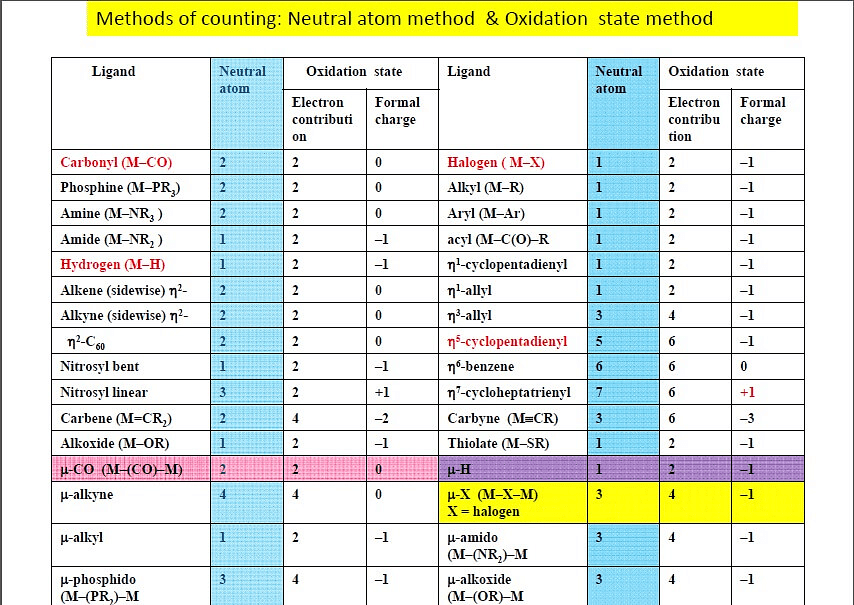
Electron Counting Methods
There are two widely used methods for electron counting of complexes - the covalent method and ionic ligand method.
Both of the two methods are applicable to all organometallic complexes and should give the same electron count.
Covalent Method
In this method, all metal-ligand bonds are considered covalent. Ligands are considered neutral in charge, and may donate either 2, 1 or zero electrons to the bond.
- For example, ligands such as CO and NH3 are considered to have filled valence and contribute 2 electrons.
- Halide and hydroxo groups, however, do not have octet structure in neutral state, and contribute 1 electron to the bonding.
- Ligands such as BF3 do not have any free electrons available, and the two electrons for bonding would come from the metal center.
Steps for covalent counting method:
- Identify the group number of the metal center.
- Identify the number of electrons contributed by the ligands.
- Identify the overall charge of the metal-ligand complex.
- At the presence of a metal-metal bond, one electron is counted towards each metal center in a bond.
- Add up the group number of the metal center and the e- count of the ligands, then take into consideration the overall charge of the complex to obtain the final electron count.
Ionic Method or Oxidation State Method
The ionic method always assigns filled valences to the ligands. For example, H group is now considered H-, as well as other groups such as halide, hydroxyl, and methyl groups.
- These groups now contribute one more electron than they do in the covalent method and oxidize the metal center when a bond is formed.
- Groups with neutral charge in octet structure, such as CO and NH3, behave the same as in valence methods.
Steps for ionic counting method:
- Determine the overall charge of the metal complex.
- Identify the charges of the ligands, and the numbers of e-s they donate.
- Determine the number of valence electrons of the metal center, so that the oxidation state of the metal and charges of the ligands balance the overall charge of the complex. (E- count of metal center = Metal atom group number + ∑(charges of ionic ligands) – overall charge of the complex)
- If a metal-metal bond is present, one bond counts for one electron for each metal atom.
- Add up the electron count of the metal center and the ligands.
Electron Counts of Some Common Ligands
| Complexes | Covalent Method | Ionic Method | Total Number of Electrons |
|---|---|---|---|
| (η5-C5H5)2Fe |
|
| 18 |
| [V(CO)7]+ |
|
| 18 |
| [Re(CO)5(PF3)]+ |
|
| 18 |
Applications of Organometallic Compounds
Organometallic Compounds have a broad range of applications in the field of chemistry. Some of them are given below-
- In some commercial chemical reactions, organometallic compounds are used as homogeneous catalysts.
- These compounds are used as stoichiometric reagents in both industrial and research-oriented chemical reactions.
- These compounds are also used in the manufacture of some semiconductors, which require the use of compounds such as trimethylgallium, trimethylaluminum, trimethylindium, and trimethyl antimony.
- They are also used in the production of light-emitting diodes (or LEDs).
- These compounds are employed in bulk hydrogenation processes such as the production of margarine.
- These compounds are used as catalysts and reagents during the synthesis of some organic compounds.
- The complexes formed from organometallic compounds are useful in the facilitation of the synthesis of many organic compounds.
The points given above emphasize the importance of organometallic compounds. However, they are also the cause of many environmental concerns due to the highly toxic nature of some of these types of compounds.
|
48 videos|92 docs|41 tests
|
FAQs on Organometallic Chemistry: Hapticity of Ligands & 18 Electron Rule - Inorganic Chemistry
| 1. What are organometallic compounds? |  |
| 2. What is hapticity in ligands? |  |
| 3. What is the 18 electron rule in organometallic chemistry? |  |
| 4. How are electron counting methods used in organometallic chemistry? |  |
| 5. What are some applications of organometallic compounds? |  |

|
Explore Courses for Chemistry exam
|

|


















