Stereo: D/L, R/S , Erythro/Threo Nomenclature of Organic Compounds | Organic Chemistry PDF Download
D/L System of Nomenclature
D/L system of Nomenclature is the most commonly used system for assigning the configuration to be given enantiomer.
- The oldest system of nomenclature of enantiomers is D,L system.
- In this system, the configuration of all the compounds was designated with respect to glyceraldehydes the configuration of which was taken as an arbitrary standard by Rosanoff (1906).
- (+) Glyceraldehyde having the OH group on the right and hydrogen on the left-CHO and-CH2OH group being on the top and the bottom respectively was arbitrarily given the configurational symbol D.
- The mirror image compound of (+) Glyceraldehyde was given the configuration L.
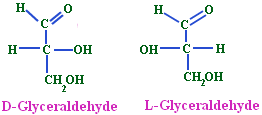
- Any compound that can be prepared from or converted into D (+) Glyceraldehyde will belong to D-series and the same is true for L-(-)-Glyceraldehyde.
- There is no change in configuration if a reaction doesn’t involve the cleavage of a bond to the chiral centre.
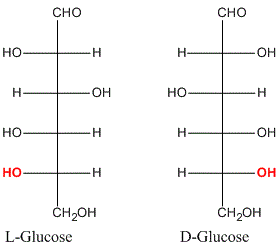

Drawbacks of D, L Nomenclature
- It indicated nothing more than relative configurations i.e. there was no way of deciding whether these stereochemical representations reflected reality.
- D, L –nomenclature creates confusion in assigning the configurations to some compounds. Tartaric acid may be assigned L configuration with respect to the bottom COOH and may be assigned D configuration with respect to the top COOH.
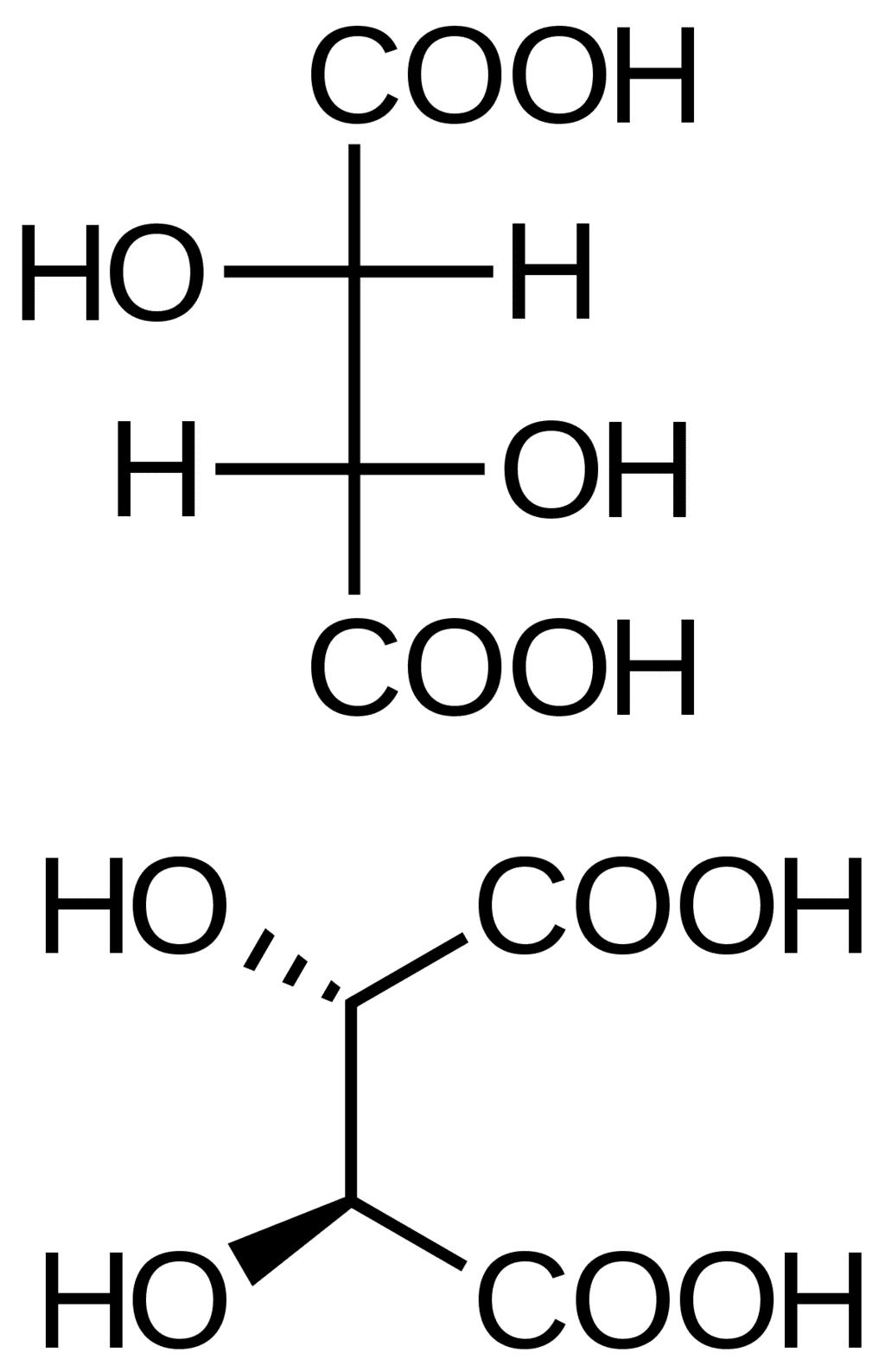 Tartaric Acid
Tartaric Acid
- The D, L system specifies the configurations of only one chiral atom. The configurations at the other chiral centers must be memorized.
- The assignment of configurations to amino acids presented difficulties which resulted in the development of amino acid nomenclature.
R,S Nomenclature
According to the Cahn-Ingold-Prelog system, there are a set of rules that can be used for unambiguously defining the stereochemical configuration of any stereocenter. In this R is for Latin rectus which means right-handed and S is for Latin sinister which means left-handed.
- An unambiguous and universally applicable system for specifying the absolute configuration of each chiral center in a molecule was devised by Cahn et al.
Rules of R, S Nomenclature
- The atom of lowest priority is always away from the observer. If it is toward the observer then the configuration is opposite to that we observe.
- Hydrogen is the lowest priority group when a lone pair is absent. When a lone pair is present it is given the lowest priority.
- Priorities are assigned on the basis of higher atomic number. If two atoms are isotopes of the same element the isotope of higher mass number has the highest priority.

- If two atoms attached to the chiral center are the same the priority is decided by applying the sequence rule to the next atoms in the groups and soon.
- For example: 2-bromobutane two-atom directly attached to the chiral center are carbon. In CH3 the second atoms are H, H, and H in C2H5 they are C, H, H. Since carbon has a higher atomic number so, C2H5 is preferred over CH3.
- If there is a double or triple bond, both double and triple bond atoms are considered to be duplicated or triplicate.
- The priority of cis alkene is higher than trans alkene.
Comparison between D, L, and R, S nomenclature:
- The D, L system of nomenclature is for the nomenclature of relative configuration whereas the R, S system is for the nomenclature of absolute configuration.
- There is no relation between these two systems of nomenclature and sign of rotation. All the D compounds may not have R and all the L compounds may not have S configurations.
Erythro and Threo Nomenclature
Erythro and threo are common terms in stereochemistry used for naming molecules with two stereogenic centers. The names derive from the saccharides erythrose and threose.
- An enantiomer of the one pair is diastereomeric with that of the other pair.
- This nomenclature is based on aldotetrose, erythrose, and threose which exist as two enantiomeric pairs.

- The Erythro isomer has similar groups at two adjacent chiral centers on the same side of the Fischer projection, while the Threo isomer has the corresponding groups on opposite sides of the Fischer projection.
- This approach for naming chiral compounds became quite general and spread outside of carbohydrates.
- The configuration of any molecule with two stereogenic centers can be classified as erythro or threo.
- For example, the following halide is said to be erythro when the Cl and Br groups on the neighboring carbon atoms are on the same side, and threo, when they are on the opposite sides:

Comparison between Erythro-threo and Meso-dl pair
For Erythro and Threo nomenclature.
- R must be equal to R
- R1 may or may not be equal to R1.
- R1 should not be equal to R4.
For Meso and dl pair
- R must be equal to R
- a must be equal to a
- b must be equal to b


Cis-Trans Nomenclature for Geometrical Isomers
cis/trans nomenclature is effective only when the alkene has two different groups on each carbon atom of the double bond and each carbon has one of the same group.
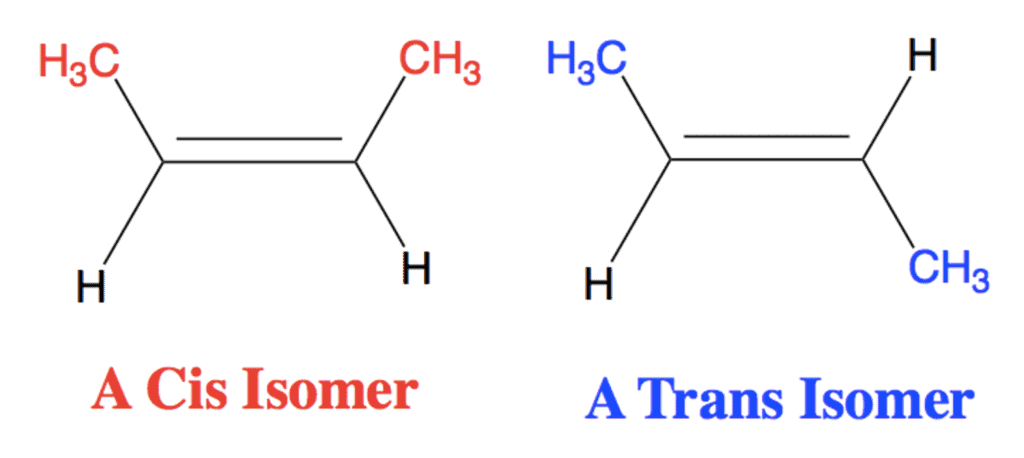
- The isomers in which the identical groups are on the same side of the double bond is called Cis and the isomer in which the identical groups are on the opposite sides is called trans.
- This type of nomenclature can be used only when two or three types of ligands are attached around the double bond.
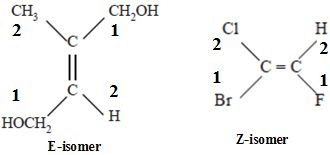
E-Z Nomenclature for Geometrical Isomers
E-Z notation is used to name geometric isomers. It is an extension of cis-trans isomer. When none or most of the groups attached to the double bond are not same then in IUPAC system geometric isomers are notified or named with E or Z.
- E-Z is based on the cahn-ingold-prelog system. In the E-Z system, the group of highest priority on each carbon atom is identified by using the sequence rules.
- If the highest priority groups are on the same side of the double bond, the configuration is Z (zusammen=together) and if they are on the opposite sides the configuration is E (entegegen=opposite).
|
35 videos|92 docs|46 tests
|
FAQs on Stereo: D/L, R/S , Erythro/Threo Nomenclature of Organic Compounds - Organic Chemistry
| 1. What is the D/L system of nomenclature? |  |
| 2. How does the R/S nomenclature work? |  |
| 3. How does the erythro and threo nomenclature work? |  |
| 4. What is the difference between erythro-threo and meso-dl pairs? |  |
| 5. How does the cis-trans nomenclature work for geometrical isomers? |  |

|
Explore Courses for Chemistry exam
|

|



















