Electromagnetic Radiation - Wave & Particle Nature | Physical Chemistry PDF Download
Electromagnetic Radiation
Newton was first person to comment on the nature of light in terms of Corpuscular. Theory of Light. According to this theory light is a stream of particles commonly known as corpuscles of light. He was able to explain reflection and refraction, the most common phenomenon of light. But the other phenomenon like diffraction and interference could not be explained on the basis of this theory.
Maxwell, in 1956 proposed that radiant energy (light) has wave characteristics. Light according to him is Electromagnetic Wave arising due to the disturbance created by electric and magnetic fields, oscillating perpendicular to each other in space. Like all other mechanical waves, it is characterized by velocity c, frequency ν, wavelength λ which are related as:
c = νλ. The value of c is constant and equal to 3 × 108 m/s.
Electromagnetic Spectrum
Electromagnetic wave or radiation is not a single wavelength radiation, but a mixture of various wavelength or frequencies. All the frequencies have same speed.
If all the components of Electromagnetic Radiation (EMR) are arranged in order of decreasing or increasing wavelengths or frequencies, the pattern obtained is known as Electromagnetic Spectrum. The following table shows all the components of light.
S. No. | Name | Wavelength | Frequency(Hz) | Source |
1. | Radio wave | 3×1014 – 3×107 | 1×105 – 1×109 | Alternating current of high frequency |
2. | Microwave | 3×107 – 6×106 | 1×109 – 5×1011 | Klystron tube |
3. | Infrared (IR) | 6×106 – 7600 | 5×1011 – 3.95×1016 | Incandescent objects |
4. | Visible | 7600 – 3800 | 3.95×1016 – 7.9×1014 | Electric bulbs, sun rays |
5. | Ultraviolet (UV) | 3800 – 150 | 7.9×1014 – 2×1016 | Sun says, are lamps with mercury vapours |
6. | X-rays | 150 – 0.1 | 2×1016 – 3×1019 | Cathode rays striking metal plate |
7. | Γ-Rays | 0.1 – 0.01 | 3×1019 – 3×1020 | Secondary effect of radioactive decay |
8. | Cosmic Rays | 0.01 – zero | 3×1020 – Infinity | Outer space |
Photoelectric Effect
It was observed by Hertz and Lenard around 1880 that when a clean metallic surface is irradiated by monochromatic light of proper frequency, electrons are emitted from it. This phenomenon of ejection of the electrons from metal surface was called as Photoelectric effect.
It was observed that if the frequency of incident radiation is below a certain minimum value (threshold) frequency),no emission takes place however high the intensity of light may be.
Another important feature observed was that the kinetic energy of the electrons emitted is independent of the Intensity of the light. The kinetic energy of the electrons increases linearly with the frequency of incident light radiation. This was highly contrary to the laws of Physics at that time i.e., the energy of the electrons should have been proportional to the intensity of the light, not on the frequency.
These features could not be properly explained on the basis of Maxwell’s concept of light i.e. light as electromagnetic wave.
In 1905, Einstein applied Planck’s quantum theory of light to account for the extraordinary features of the photoelectric effect. He introduced a new concept that light shows dual nature. In phenomenon like reflection, refraction and diffraction it shows wave nature and in phenomenon like photoelectric effects, it shows particle nature. According to the particle nature, the energy of the light is carried in discrete units whose magnitude is proportional to the frequency of the light wave. These units were called as photons (or quanta).
According to Einstein, when a quantum of light (photon) strikes a metal surface, it imparts its energy to the electrons in the metal. In order for an electron to escape from the surface of the metal, it must overcomes the attractive force of the positive ions in the metal. So a part of the photon’s energy is absorbed by the metal surface to release the electron, this is known as work function of the surface and is denoted by φ. The remaining part of the energy of the photon goes into the kinetic energy of the electron emitted. If E is the energy of the photon, KE is the kinetic energy of the electron and φ be the work function of the metal then we have;


Also, if m be the mass and v be the velocity of the electron ejected then
KE = 1/2 mv2 = h(ν – ν0).
Wave-Particle Duality
We have heard about the nature of light and the characters it displays. Interference, reflection, refraction and diffraction are some of the characteristics. Wave-Particle Duality helps us to understand the particle and wave nature of light.
Based on the idea that light and all other electromagnetic radiation may be considered a particle or a wave nature, in 1923 physicists Louis De Broglie suggested that the same kind of duality must be applicable to the matter. He proposed that any particle of matter having momentum (p) Has an associated wavelength (λ).
De Broglie Wavelength formula is given by λ= h/p Where, h is the Planck constant for a particle of momentum mv, the wavelength is given by λ=h/mv. This equation is also applicable for photons too.
Early in this century, De Broglie began to realize the extent to which both models describe the same phenomena. Waves can be described as particles; particles, as waves. Diffraction and interference phenomena reveal a wave-like character in light, but the photoelectric effect, absorption and emission by atoms show light to have a particle-like character as well. Electrons have mass but can be diffracted like caves. Nature reveals a particle duality and ambiguity uncharacteristic of science. While the meaning of this wave-particle duality remains a subject of intense debate, many physicists now accept the Bohr complement- yarn principle. The two models exclude one another, yet both are necessary for a complete description of nature.e.
According to Planck’s Hypothesis of the Quantum Theory, the energy is emitted in quanta, which are little packets of energy. He states that energy emitted is related to the frequency of the emitted light and this can be considered as Wave-Particle Duality definition. According to Planck’s hypothesis, the quantum energy is related to the frequency by the equation E = hν.
Observing a light is one of the easiest ways to prove the duality between a particle and a wave. Since light is similar to waves, it is able to diffract, refract, and interface, etc.
Black Body Radiation
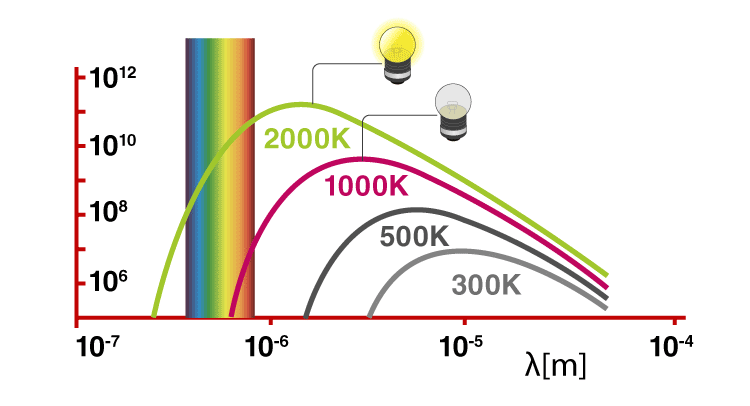
Solids, when heated, emit radiation varying over a wide range of wavelengths. For example: when we heat solid colour, changes continue with a further increase in temperature. This change in colour happens from a lower frequency region to a higher frequency region as the temperature increases. For example, in many cases, it changes from red to blue. An ideal body which can emit and absorb radiation of all frequencies is called a black body. The radiation emitted by such bodies is called black body radiation.
Thus, we can say that variation of frequency for black body radiation depends on the temperature. At a given temperature, the intensity of radiation is found to increase with an increase in the wavelength of radiation which increases to a maximum value and then decreases with an increase in the wavelength. This phenomenon couldn’t be explained with the help of Maxwell’s suggestions. Hence, Planck proposed Planck’s quantum theory to explain this phenomenon.
Planck’s quantum theory
According to Planck’s quantum theory,
- Different atoms and molecules can emit or absorb energy in discrete quantities only. The smallest amount of energy that can be emitted or absorbed in the form of electromagnetic radiation is known as quantum.
- The energy of the radiation absorbed or emitted is directly proportional to the frequency of the radiation.
Meanwhile, the energy of radiation is expressed in terms of frequency as,
E = h ν
Where,
E = Energy of the radiation
h = Planck’s constant (6.626×10–34 J.s)
ν= Frequency of radiation
Interestingly, Planck has also concluded that these were only an aspect of the processes of absorption and emission of radiation. They had nothing to do with the physical reality of the radiation itself. Later in the year 1905, famous German physicist, Albert Einstein also reinterpreted Planck’s theory to further explain the photoelectric effect. He was of the opinion that if some source of light was focused on certain materials, they can eject electrons from the material. Basically, Planck’s work led Einstein in determining that light exists in discrete quanta of energy, or photons.
|
83 videos|142 docs|67 tests
|
FAQs on Electromagnetic Radiation - Wave & Particle Nature - Physical Chemistry
| 1. What is electromagnetic radiation? |  |
| 2. What is wave-particle duality? |  |
| 3. How does electromagnetic radiation exhibit wave-like behavior? |  |
| 4. How does electromagnetic radiation exhibit particle-like behavior? |  |
| 5. How does the wave-particle duality of electromagnetic radiation impact our understanding of light and matter? |  |

|
Explore Courses for Chemistry exam
|

|


















