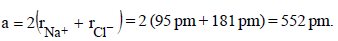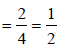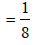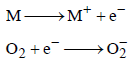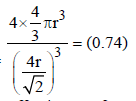Test: MCQs (One or More Correct Option): The Solid State & Surface Chemistry | JEE Advanced - JEE MCQ
8 Questions MCQ Test - Test: MCQs (One or More Correct Option): The Solid State & Surface Chemistry | JEE Advanced
Which of the following statement(s) is (are) correct?(1998 - 2 Marks)
The correct statement(s) regarding defects in solids is (are) (2009S)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The correct statement(s) pertaining to the adsorption of agas on a solid surface is (are) (2011)
Choose the correct reason(s) for the stability of the lyophobiccolloidal particles. (2012)
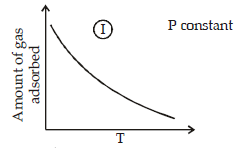
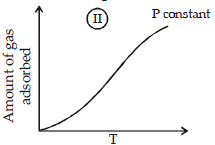
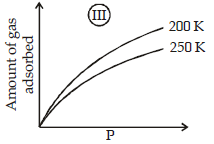
The given graphs/data I, II, III and IV represent general trends observed for different physisorption and chemisorption processes under mild conditions of temperature and presure.
Which of the following choice(s) about I, II, III and IV is (are) correct ? (2012)

If the unit cell of a mineral has cubic close packed (ccp) array of oxygen atoms with m fraction of octahedral holes occupied by aluminium ions and n fraction of tetrahedral holes occupied by magnesium ions, m and n, respectively, are (JEE Adv. 2015)
When O2 is adsorbed on a metallic surface, electron transferoccurs from the metal to O2. The true statement(s) regardingthis adsorption is(are) (JEE Adv. 2015)
The CORRECT statement(s) for cubic close packed (ccp)three dimensional structure is (are) (JEE Adv. 2016)