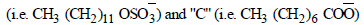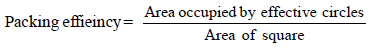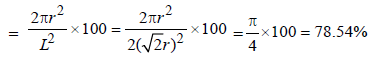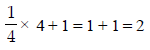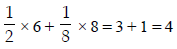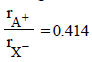JEE Advanced (Single Correct MCQs): The Solid State & Surface Chemistry - JEE MCQ
14 Questions MCQ Test - JEE Advanced (Single Correct MCQs): The Solid State & Surface Chemistry
CsBr has bcc structure with edge length 4.3. The shortestinter ionic distance in between Cs+ and Br– is : (1995S)
The coordination number of a metal crystallizing in ahexagonal close-packed structure is (1999 - 2 Marks)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a solid ‘AB’ having the NaCl structure, ‘A’ atoms occupythe corners of the cubic unit cell. If all the face-centeredatoms along one of the axes are removed, then the resultantstoichiometry of the solid is (2001S)
A substance AxBy crystallizes in a face centred cubic (FCC)lattice in which atoms 'A' occupy each corner of the cubeand atoms 'B' occupy the centres of each face of the cube.Identify the correct composition of the substance AxBy (2002S)
Rate of physiorption increases with (2003S)
Adsorption of gases on solid surface is generally exothermicbecause (2004S)
In which of the following crystals alternate tetrahedral voidsare occupied? (2005S)
Lyophilic sols are (2005S)
Among the following, the surfactant that will form micellesin aqueous solution at the lowest molar concentration atambient condition is : – (2008S)
Among the electrolytes Na2SO4, CaCl2, Al2(SO4)3 and NH4Cl, the most effective coagulating agent for Sb2S3 solis (2009S)
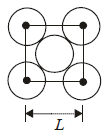
The packing efficiency of the two-dimensional square unit cell shown below is :
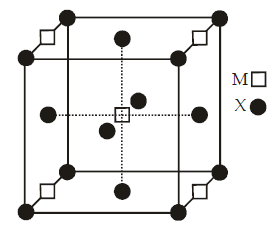
A compound MpXq has cubic close packing (ccp) arrangement of X. Its unit cell structure is shown below.
The empirical formula of the compound is (2012 - I)
The arrangement of X– ions around A+ ion in solid AX is given in the figure (not drawn to scale). If the radius of X– is 250 pm, the radius of A+ is
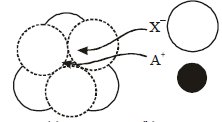
Methylene blue, from its aqueous solution, is adsorbed onactivated charcoal at 25°C. For this process, the correctstatement is (JEE Adv. 2013)


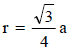
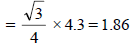
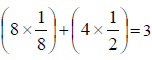
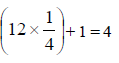
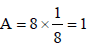
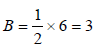
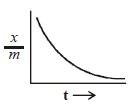
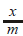 = amt of gas adsorbed per unit
= amt of gas adsorbed per unit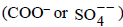 ion
ion