Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - JEE MCQ
Test Description
8 Questions MCQ Test - Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced for JEE 2025 is part of JEE preparation. The Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced questions and answers have been prepared
according to the JEE exam syllabus.The Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced MCQs are made for JEE 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced below.
Solutions of Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced questions in English are available as part of our course for JEE & Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced solutions in
Hindi for JEE course.
Download more important topics, notes, lectures and mock test series for JEE Exam by signing up for free. Attempt Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced | 8 questions in 10 minutes | Mock test for JEE preparation | Free important questions MCQ to study for JEE Exam | Download free PDF with solutions
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 1
An isotone of  is : (1984 - 1 Mark)
is : (1984 - 1 Mark)
 is : (1984 - 1 Mark)
is : (1984 - 1 Mark)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 1
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 2
Many elements have non-integral atomic masses because : (1984 - 1 Mark)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 2
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 3
When alpha particles are sent through a thin metal foil, most of them go straight through the foil because : (1984 - 1 Mark)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 3
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 4
The sum of the number of neutrons and proton in the isotope of hydrogen is : (1986 - 1 Mark)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 4
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 5
The energy of an electron in the first Bohr orbit of H atom is –13.6 eV. The possible energy value(s) of the excited state(s) for electrons in Bohr orbits of hydrogen is (are) (1998 - 2 Marks)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 5
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 6
Which of the following satement(s) is (are) correct? (1998 - 2 Marks)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 6
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 7
Decrease in atomic number is observed during (1998 - 2 Marks)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 7
*Multiple options can be correct
Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 8
Ground state electronic configuration of nitrogen atom can be represented by (1999)
Detailed Solution for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced - Question 8
Information about Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced Page
In this test you can find the Exam questions for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: MCQs (One or More Correct Option): Structure of Atom | JEE Advanced, EduRev gives you an ample number of Online tests for practice
Download as PDF


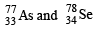 have same number of neutrons
have same number of neutrons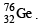


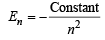
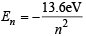 of the given values of energy,, only – 3.4 eV and – 1.5 eV can be obtained by substituting n =2 and 3 respectively in the above expression.
of the given values of energy,, only – 3.4 eV and – 1.5 eV can be obtained by substituting n =2 and 3 respectively in the above expression.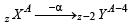 (α - emission) ;
(α - emission) ;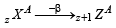 (β - emission);
(β - emission);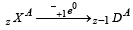 (positron - emission);
(positron - emission);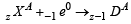 (electron - capture)
(electron - capture)














