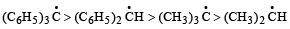Test: JEE Main 35 Year PYQs- Organic Chemistry Some Basic Principles & Technique - JEE MCQ
30 Questions MCQ Test - Test: JEE Main 35 Year PYQs- Organic Chemistry Some Basic Principles & Technique
Read the following Statement-1(Asseration) and Statement -2 (Reason) and answer as per the options given below :
Q.
Statement -1: Aryl halides undergo nucleophilic substitution with ease.
Statement -2:The carbon-halogen bond in aryl halides has partial double bond character.
Statement -1: Phenol is more reactive than benzene towards electrophilic substitution reactions.
Statement-2:In the case of phenol, the intermediate carbocation is more resonance stabilized.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
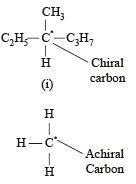
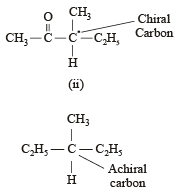
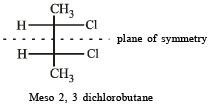
Statement -1: Molecules that are not superimpossable on their mirror images are chiral.
Statement -2: All chiral molecules have chiral centres.
Arrangement of (CH3)3C –, (CH3)2CH –, CH3 – CH2 – when attached to benzyl or an unsaturated group in increasing order of inductive effect is
A similarity between optical and geometrical isomerism is that
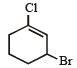
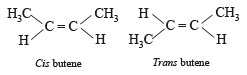
Which of the following does not show geometrical isomerism?
The functional group, which is found in amino acid is
Which of the following compounds has wrong IUPAC name?
The IUPAC name of CH3COCH(CH3), is
In which of the following species is the underlined carbon having sp3 hybridisation?
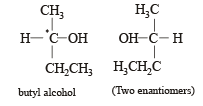
Racemic mixture is formed by mixing two
Following types of compounds (as I, II)
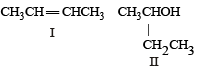
are studied in terms of isomerism in:
The reaction:
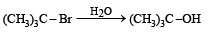
In the anion HCOO– the two carbon-oxygen bonds are found to be of equal length. what is the reason for it ?
The general formula CnH2nO2 could be for open chain
Among the following four structures I to IV,
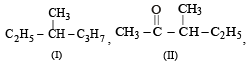
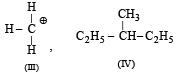
it is true that
Which one of the following has the minimum boiling point ?
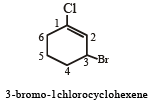
The IUPAC name of the compound is 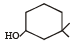
Which one of the following does not have sp2 hybridized carbon ?
Which of the following will have a mesoisomer also?
Rate of the reaction
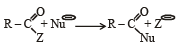
is fastest when Z is
Amongst the following compounds, the optically active alkane having lowest molecular mass is
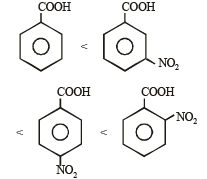
Consider the acidity of the carboxylic acids :
(a) PhCOOH
(b) o-NO2C6H4COOH
(c) p-NO2C6H4COOH
(d) m-NO2C6H4COOH
Which of the following order is correct ?
Which of the following is the strongest base ?
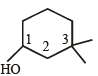
Which of the following compounds is not chiral?
Due to the presence of an unpaired electron, free radicals are:
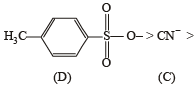
The decreasing order of nucleophilicity among the nucleophiles
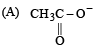
(B) CH3O-
(C) CN-
The reaction
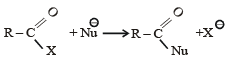
is fastest when X is
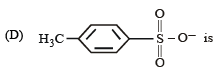
The IUPAC name of the compound shown below is :
The increasing order of stability of the following free radicals is


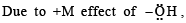 its in termediate carbocation is more stable than the one in benzene.
its in termediate carbocation is more stable than the one in benzene.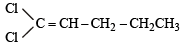 does not show geometrical isomerism due to presence of two similar Cl atoms on the same C-atom. Geometrical isomerism is shown by compounds in which the groups/atoms attached to C = C are different.
does not show geometrical isomerism due to presence of two similar Cl atoms on the same C-atom. Geometrical isomerism is shown by compounds in which the groups/atoms attached to C = C are different.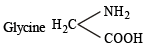
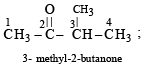
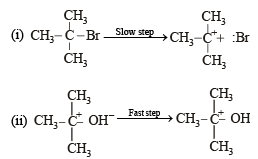

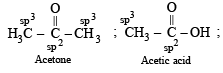
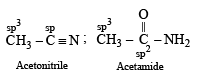
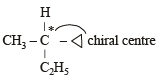
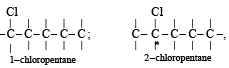
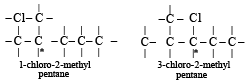
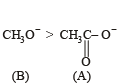
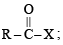 when X is Cl the C–X bond is more polar and ionic which leaves the compound more reactive for nucleophilic substitution reaction.
when X is Cl the C–X bond is more polar and ionic which leaves the compound more reactive for nucleophilic substitution reaction.