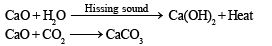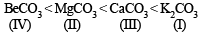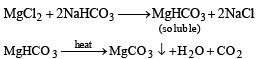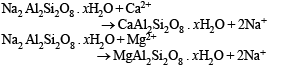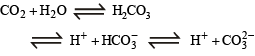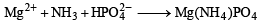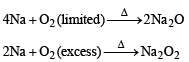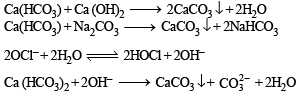Test: MCQs (One or More Correct Option): The s-Block Elements | JEE Advanced - JEE MCQ
23 Questions MCQ Test - Test: MCQs (One or More Correct Option): The s-Block Elements | JEE Advanced
A substance absorbs CO2 and voilently reacts with water.The substance is
HCl is added to following oxides. Which one would give H2O2?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Calcium is obtained by
A solution of sodium metal in liquid ammonia is strongly reducing due to the presence of
Heavy water is
The hydration energy of Mg++ is larger than that of :
The oxide that gives hydrogen peroxide on treatment with a dilute acid is :
Molecular formula of Glauber ’s salt is :
Hydrogen gas will not reduce :
The pair of compounds which can not exist together in solution is :
The metallic lustre exhibited by sodium is explained by
The volume strength of 1.5 N H2O2 solution is
The following compounds have been arranged in order of their increasing thermal stabilities. Identify the correct order.
K2CO3 (I) MgCO3 (II) CaCO3 (III) BeCO3 (IV)
The set representing the correct order of first ionization potential is
A sodium salt on treatment with MgCl2 gives white precipitate only on heating. The anion of the sodium salt is
Hydrogen peroxide in its reaction with KIO4 and NH2OH respectively, is acting as a
When zeolite, which is hydrated sodium aluminium silicate, is treated with hard water the sodium ions are exchanged with
The species that do not contain peroxide ions are
Highly pure dilute solution of sodium in liquid ammonia
The species present in solution when CO2 is dissolved in water are
MgSO4 on reaction with NH4OH and Na2HPO4 forms a white crystalline precipitate. What is its formula?
The compound(s) formed upon combustion of sodium metal in excess air is (are)
The reagent(s) used for softening the temporary hardness of water is (are)