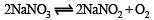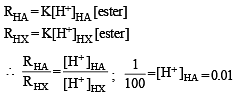Test: MCQs (One or More Correct Option): Equilibrium | JEE Advanced - JEE MCQ
12 Questions MCQ Test - Test: MCQs (One or More Correct Option): Equilibrium | JEE Advanced
For the gas phase reaction :

carried out in a vessel, the equilibrium concentration of C2H4 can be increased by :

When NaNO3 is heated in a closed vessel, oxygen is liberated and NaNO2 is left behind. At equilibrium.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The equilibrium :

is attained at 25ºC in a closed container and an inert gas, helium is introduced. Which of the following statements are correct?

For the reaction :

The forward reaction at constant temperature is favoured by
For the reaction  at a given temperature the equilibrium amount of CO2(g) can be increased by
at a given temperature the equilibrium amount of CO2(g) can be increased by
Which of the following statements(s) is (are) correct?
A buffer solution can be prepared from a mixture of
Aqueous solutions of HNO3 , KOH, CH3COOH and CH3COONa of identical concentrations are provided. The pair(s) of solutions which form a buffer upon mixing is(are)
The initial rate of hydrolysis of methyl acetate (1M) by a weak acid (HA, 1M) is 1/100th of that of a strong acid (HX, 1M), at 25°C. The Ka of HA is
The Ksp of Ag2CrO4 is 1.1 × 10–12 at 298 K. The solubility (in mol/L) of Ag2CrO4 in a 0.1 M AgNO3 solution is
The thermal dissociation equilibrium of CaCO3(s) is studied under different conditions

For this equilibrium, the correct statement(s) is(are)
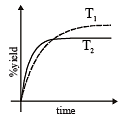
The %yield of ammonia as a function of time in the reaction is given below
is given below

If this reaction is conducted at (P, T2), with T2 > T1, the %yield of ammonia as a function of time is represented by