Test: MCQs (One or More Correct Option): The d- and f-Block Elements & Coordination Compounds | JEE Advanced - JEE MCQ
15 Questions MCQ Test - Test: MCQs (One or More Correct Option): The d- and f-Block Elements & Coordination Compounds | JEE Advanced
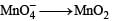
Potassium manganate (K2MnO4) is formed when
The aqueous solutions of the following salts will be coloured in the case of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Among the following ions which one has the highest paramagnetism?
Which of the following alloys contains(s) Cu and Zn?
In nitroprusside ion the iron and NO exist as FeII and NO+ rather than FeIII and NO. These forms can be differentiated by
Addition of high proportions of manganese makes steel useful in making rails of railroads, because manganese
If the bond length of CO bond in carbon monoxide is 1.128Å, then what is the value of CO bond length in Fe(CO)5?
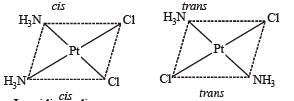
The compound(s) that exhibit(s) geometrical isomerism is (are)
Reduction of the metal centre in aqueous permanganate ion involves
The equilibrium
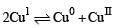
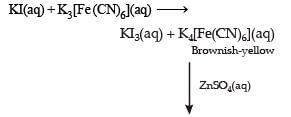
For the given aqueous reactions, which of the statement (s) is (are) true?
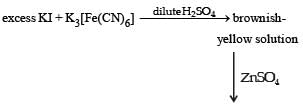

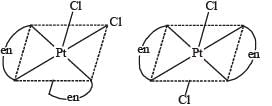
The pair(s) of coordination complexes/ions exhibiting the same kind of isomerism is(are)
The pair (s) of reagents that yield paramagnetic species is/are
The correct statement(s) about Cr2+ and Mn3+ is(are) [Atomic numbers of Cr = 24 and Mn = 25]
Fe3+ is reduced to Fe2+ by using


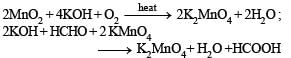
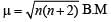
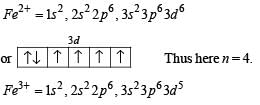


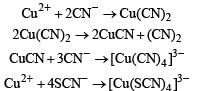
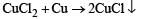
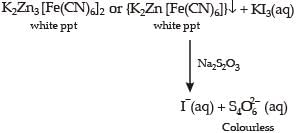
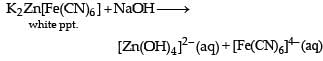
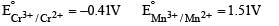 Above E° values explains reducing nature of Cr2+ and oxidizing behaviour of Mn3+.
Above E° values explains reducing nature of Cr2+ and oxidizing behaviour of Mn3+.














