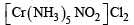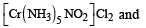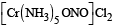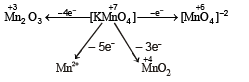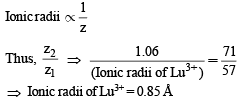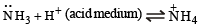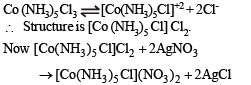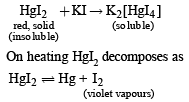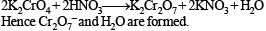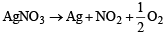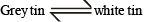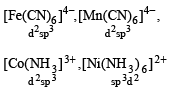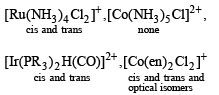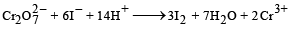Test: 35 Year JEE Previous Year Questions: The d- and f-Block Elements & Coordination Compounds - JEE MCQ
30 Questions MCQ Test - Test: 35 Year JEE Previous Year Questions: The d- and f-Block Elements & Coordination Compounds
A square planar complex is formed by hybridisation of which atomic orbitals?
The type of isomerism present in nitropentammine chromium (III) chloride is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
CH3 – Mg – Br is an organo metallic compound due to
Most common oxidation states of Ce (cerium) are
Arrange Ce+3, La+3, Pm+3 and Yb+3 in increasing order of their ionic radii.
Which of the following ions has the maximum magnetic moment?
The most stable ion is
When KMnO4 acts as an oxidising agent and ultimately forms [MnO4]–2, MnO2, Mn2O3, Mn+2 then the number of electrons transferred in each case respectively is
The radius of La3+ (Atomic number of La = 57) is 1.06Å.
Which one of the following given values will be closest to the radius of Lu3+ (Atomic number of Lu = 71) ?
Ammonia forms the complex ion [Cu(NH3)4]2+ with copper ions in alkaline solutions but not in acidic solutions. What is the reason for it ?
One mole of the complex compound Co(NH3)5Cl3, gives 3 moles of ions on dissolution in water. One mole of the same complex reacts with two moles of AgNO3 solution to yield two moles of AgCl (s). The structure of the complex is
In the coordination compound, K4[Ni(CN)4], the oxidation state of nickel is
A red solid is insoluble in water. However it becomes soluble if some KI is added to water. Heating the red solid in a test tube results in liberation of some violet coloured fumes and droplets of a metal appear on the cooler parts of the test tube. The red solid is
A reduction in atomic size with increase in atomic number is a characteristic of elements of
What would happen when a solution of potassium chromate is treated with an excess of dilute nitric acid?
Which one of the following nitrates will leave behind a metal on strong heating ?
Of the following outer electronic configurations of atoms, the highest oxidation state is achieved by which one of them ?
The soldiers of Napolean army while at Alps during freezing winter suffered a serious problem as regards to the tin buttons of their uniforms. White metallic tin buttons got converted to grey power. This transformation is related to
Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by CN– ion towards metal species is
The coordination number of a central metal atom in a complex is determined by
Which one of the following complexes is an outer orbital complex ?
(Atomic nos. : Mn = 25; Fe = 26; Co = 27, Ni = 28)
Coordination compounds have great importance in biological systems. In this context which of the following statements is incorrect ?
Cerium (Z = 58) is an important member of the lanthanoids. Which of the following statements about cerium is incorrect?
Which one of the following has largest number of isomers ?
The correct order of magnetic moments (spin only values in B.M.) anong is
(Atomic nos. : Mn = 25, Fe = 26, Co = 27)
The oxidation state Cr in [Cr (NH3)4 Cl2]+ is
Heating mixture of Cu2O and Cu2S will give
The oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is:
Calomel (Hg2Cl2) on reaction with ammonium hydroxide gives
The lanthanide contraction is responsible for the fact that


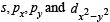 atomic orbitals
atomic orbitals