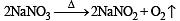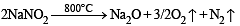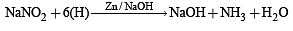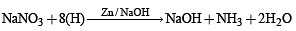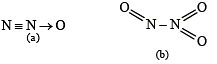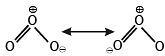Test: MCQs (One or More Correct Option): The p-Block Elements | JEE Advanced - JEE MCQ
19 Questions MCQ Test - Test: MCQs (One or More Correct Option): The p-Block Elements | JEE Advanced
In the electrolysis of alumina, cryolite is added to :
Nitrogen(I) oxide is produced by :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The compounds used as refrigerant are
The major role of fluorspar (CaF2), which is added in small quantities in the electrolytic reduction of alumina dissolved in fused cryolite (Na3AlF6), is
The material used in the solar cells contains
Sodium nitrate decomposes above 800°C to give
White phosphorus (P4) has
Ammonia, on reaction with hypochlorite anion, can form
A solution of colourless salt H on boiling with excess NaOH produces a non-flammable gas. The gas evolution ceases after sometime. Upon addition of Zn dust to the same solution, the gas evolution restarts. The colourless salt (s) H is (are)
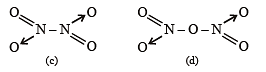
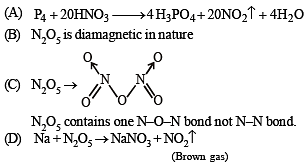
The nitrogen oxide(s) that contain(s) N-N bond(s) is(are)
Which of the following halides react(s) with AgNO3(aq) to give a precipitate that dissolves in Na2S2O3(aq)?
With respect to graphite and diamond, which of the statement(s) given below is (are) correct ?
The correct statement(s) about O3 is(are)
For the reaction
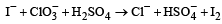
The correct statement(s) for orthoboric acid is/are
The correct statement(s) regarding,
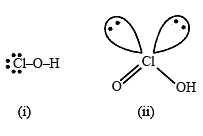
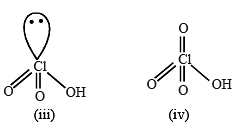
(i) HClO, (ii) HClO2, (iii) HClO3 and (iv) HClO4, is(are)
Under hydrolytic conditions, the compounds used for preparation of linear polymer and for chain termination, respectively, are
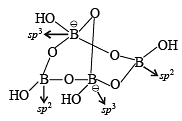
The crystalline form of borax has
The nitrogen containing compound produced in the reaction of HNO3 with P4O10