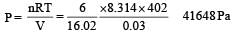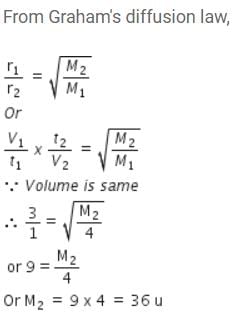31 Year NEET Previous Year Questions: States of Matter - 1 (Old NCERT) - NEET MCQ
20 Questions MCQ Test - 31 Year NEET Previous Year Questions: States of Matter - 1 (Old NCERT)
Choose the correct option for the total pressure (in atm.) in a mixture of 4 g O2 and 2 g H2 confined in a total volume of one litre at 0°C is:
[Given R=0.082 L atm mol-1 K-1, T=273 K] [2021]
[Given R=0.082 L atm mol-1 K-1, T=273 K] [2021]
A mixture of N2 and Ar gases in a cylinder contains 7 g of N2 and 8 g of Ar. If the total pressure of the mixture of the gases in the cylinder is 27 bar, the partial pressure of N2 is: [2020]
[Use atomic masses (in g mol-1): N = 14, Ar = 40]
[Use atomic masses (in g mol-1): N = 14, Ar = 40]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
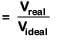
A gas at 350 K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions. The correct option about the gas and its compressibility factor (Z) is : [2019]
What is the density of N2 gas at 227°C and 5.00 atm pressure? (R = 0.0821 atm K–1 mol–1) [NEET Kar. 2013]
Maximum deviation from ideal gas is expected from : [NEET 2013]
Dipole-induced dipole interactions are present in which of the following pairs : [NEET 2013]
50 mL of each gas A and of gas B takes 150 and 200 seconds respectively for effusing through a pin hole under the similar condition. If molecular mass of gas B is 36, the molecular mass of gas A will be : [2012]
A bubble of air is underwater at temperature 15°C and the pressure 1.5 bar. If the bubble rises to the surface where the temperature is 25°C and the pressure is 1.0 bar, what will happen to the volume of the bubble ? [2011M]
Which of the following is correct option for free expansion of an ideal gas under adiabatic condition ? [2011]
Two gases A and B having the same volume diffuse through a porous partition in 20 and 10 seconds respectively. The molecular mass of A is 49 u. Molecular mass of B will be : [2011]
A gaseous mixture was prepared by taking equal mole of CO and N2. If the total pressure of the mixture was found 1 atmosphere, the partial pressure of the nitrogen (N2) in the mixture is : [2011]
By what factor does the average velocity of a gaseous molecule increase when the temperature (in Kelvin) is doubled ? [2011]
Three moles of an idea l gas expanded spontaneously into vacuum. The work done will be :[2010]
If a gas expan ds at constan t temperature, it indicates that : [2008]
The surface tension of which of the following liquid is maximum? [2005]
Van der Waal's real gas, act as an ideal gas, at which conditions? [2002]
Which of the following expressions correctly represents the relationship between the average molar kinetic energy, 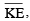 of CO and N2 molecules at the same temperature ?
of CO and N2 molecules at the same temperature ?
The pressure exerted by 6.0g of methane gas in a 0.03 m3 vessel at 129°C is (Atomic masses : C = 12.01, H = 1.01 and R = 8.314 JK–1 mol –1)
certain gas takes three times as long to effuse out as helium. Its molecular mass will be
For real gases van der Waals equation is written as
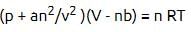
where ‘a’ and ‘b’ are van der Waals constants.
Two sets of gases are :
(I) O2, CO2, H2 and He
(II) CH4, O2 and H2
The gases given in set-I in increasing order of ‘b’ and gases given in set-II in decreasing order of ‘a’, are arranged below. Select the correct order from the following :



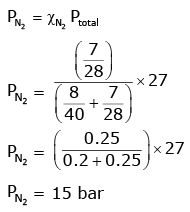
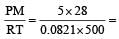 3.41g/ml
3.41g/ml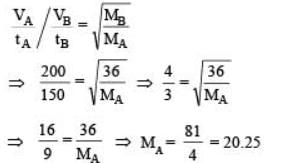
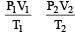
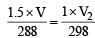
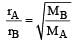
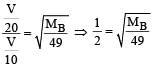
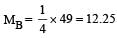
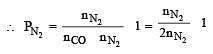
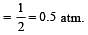
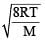
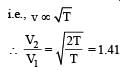
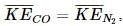 because
because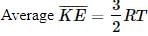 It does not depend upon the nature of the gas.
It does not depend upon the nature of the gas.