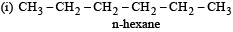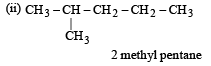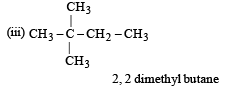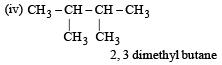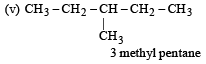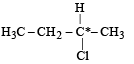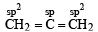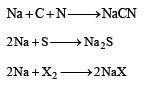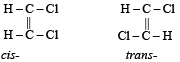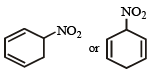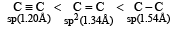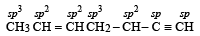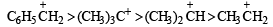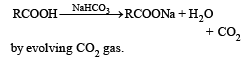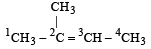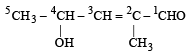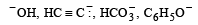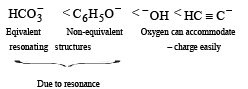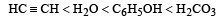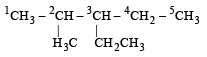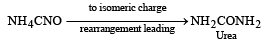31 Year NEET Questions: Some Basic Principles And Techniques- 4 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Questions: Some Basic Principles And Techniques- 4
How many chain isomers could be obtained from the alkane C6 H14 ? [1988]
Which of the following is an optically active compound ? [1988]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The Cl – C – Cl angle in 1,1,2,2- tetrachloroethene and tetrachloromethane respectively will be about[1988]
Which of the following possesses a sp-carbon in its structure ? [1989]
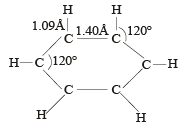
Cyclic hydrocarbon ‘A’ has all the carbon and hydrogen atoms in a single plane. All the carbon carbon bonds have the same length, less than 1.54 Å, but more than 1.34 Å. The C – C – C bond angle will be [1989]
Lassaigne’s test is used in qualitative analysis to detect[1989]
Which one of the following can exhibit cis-trans isomerism ? [1989]
Kjeldahl’s method is used in the estimation of
An organic compound X (molecular formula C6H7O2N ) has six carbon atoms in a ring system, two double bonds and a nitro group as substituent, X is [1990]
In sodium fusion test of organic compounds, the nitrogen of the organic compound is converted into[1991]
The shortest C – C bond distance is found in
An sp3 hybrid orbital contains [1991]
A straight chain hydrocarbon has the molecular formula C8H10. The hybridization of the carbon atoms from one end of the chain to the other are respectively sp3, sp2, sp2, sp3, sp2, sp2, sp and sp. The structural formula of the hydrocarbon would be : [1991]
Isomers of a substance must have the same
Which of the following is the most stable carbocation (carboniumion) ? [1991]
A is a lighter phenol and B is an aromatic carboxylic acid. Separation of a mixture of A and B can be carried out easily by using a solution of
2-Methyl 2-butene will be represented as [1992]
The IUPAC name of [1992]
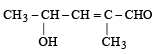
When the hybridization state of carbon atom changes from sp3 to sp2 and finally to sp, the angle between the hybridized orbitals [1993]
The most reactive compound for electrophilic nitration is [1992]
Which is the correct symbol relating the two Kekule structures of benzene ? [1993]
The restricted rotation about carbon carbon double bond in 2-butene is due to [1993]
An important chemical method to resolve a racemic mixture makes use of the formation of [1994]
The process of separation of a racemic modification into d and ℓ -enantiomers is called
Correct increasing order of acidity is as follows:
An example of electrophilic substitution reaction is[1994]
Which of the following IUPAC names is correct for the compound? [1994]
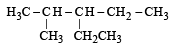
Lassaigne’s test for the detection of nitrogen fails in[1994]
The most suitable method for separtion of a 1 : 1 mixture of ortho and para nitrophenols is [1994]
The first organic compound, synthesized in the laboratory, was [1995]