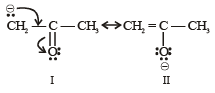31 Year NEET Questions: Some Basic Principles And Techniques- 3 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Questions: Some Basic Principles And Techniques- 3
The number of possible isomers of the compound with molecular formula C7 H8O is [1995]
The IUPAC name of [1996]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Huckel's rule states that a monocyclic conjugated compound will be aromatic if it contains [1996]
Which of the following will not show cis-trans isomerism? [1996]
Which of the following will exhibit chirality? [1996]
Decreasing order of reactivity to wards nucleophilic addition to carbonyl group among cyclopentanone, 3-pentanone and n-pentanal is [1996]
Which one of the following is a technique most suitable for purification of cyclohexanone from a mixture containing benzoic acid, isoamyl alcohol, cyclohexane and cyclohexanone? [1997]
Tautomerism will be exhibited by [1997]
The reaction :
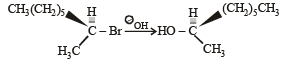
is described as [1997]
Which one of these is not compatible with arenes? [1997]
The most stable conformation of n-butane is
Among the following compounds (I - III), the ease of their reaction with electrophiles is, [1997]
IUPAC name for the compound [1998]
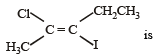
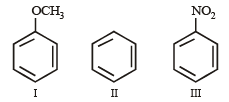
Which one of the following compounds will be most easily attacked by an electrophile? [1998]
Which of the following compounds is not chiral? [1998]
Which one of the following compounds is resistant to nucleophilic attack by hydroxyl ions?
Which one of the following orders is correct regarding the —I effect of the substituents ?
The correct structure of trans-2 hexenal is [1999]
The structural formula of a compoun d is CH3 – CH = C = CH2. The types of hybridization at the four carbons from left to right are
Which is a chiral molecule? [1999]
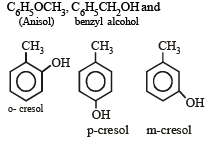
Consider the following phenols :
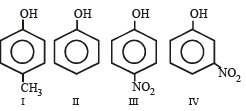
The decreasing order of acidity of the above phenols is [1999]
Correct order of stability is : [2000]
Which of the following compounds reacts slower in electrophilic substitution? [2000]
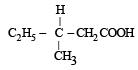
A compound of molecular formula of C7H16 shows optical isomerism, compound will be
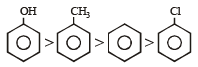
The correct order of acidic strength for following compounds will be [2001]

In steam distillation of toluene, the pressure for toluene in vapour is [2001]
The incorrect IUPAC name is [2001]
IUPAC name of the following is [2002]
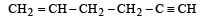
Geometrical isomers differ in [2002]


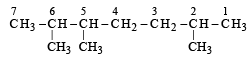
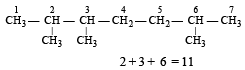
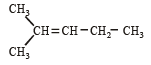
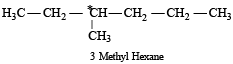
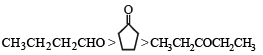
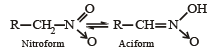
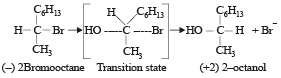

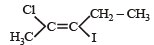
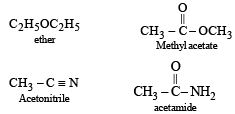
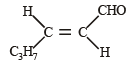
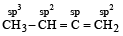
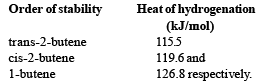
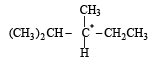

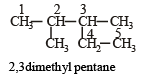
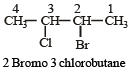
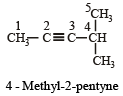
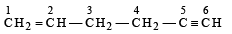
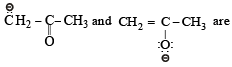 [2002]
[2002]