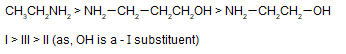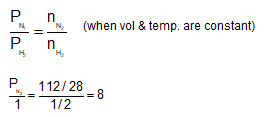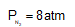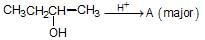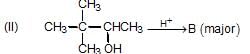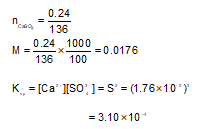Mock Test - 2 - Class 12 MCQ
30 Questions MCQ Test - Mock Test - 2
Of the following acids
I. Hypophosphorous acid
II. Orthophosphorous acid
III. Caro’s acid
IV. Glycine
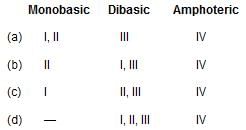
I. Hypophosphorous acid
II. Orthophosphorous acid
III. Caro’s acid
IV. Glycine

Cationic addition polymerisation reaction initiated by an acid is maximum in
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Magnetic moments of Cr(Z = 24), Mn(Z = 25) and Fe(Z = 26) are x, y and z respectively. Hence,
Silica is added to roasted copper ore during smelting in order to remove
16 g of an ideal gas SOx occupies 5.6 L at STP. The value of x for this gas is
Which of the following pairs of structures is an example of diastereomers ?
When a certain conductivity cell was filled with 0.01 M solution of KCl, it had a resistance of 160 ohm at 250C and when filled with 0.005 M NaOH, it had a resistance of 190 ohm. If specific resistance of KCl solution is 700 ohm cm, specific conductance (ohm—1 cm—1) of NaOH solution is
Arrange the following compounds in decreasing order of basicity
I. ethylamine
II. 2-amino ethanol
III. 3-amino-1-propanol
One gram of hydrogen and 112 g of nitrogen are enclosed in two separate containers each of volume 5 L at 270C. If the pressure of hydrogen is 1 atm, the pressure of nitrogen would be
A buffer solution contains 100 mL of 0.01 M CH3COOH and 200 mL of 0.02 M CH2COONa. 700 mL of water is added to this solution . pH before and after dilution are (pKa = 4.74)
Following is the concentration cell in which electrode is reversible with respect to anion
Pt(Cl2)|Cl—(C1)||Cl—(C2)|Pt(Cl2)
1 bar 1 bar
The cell reaction is spontaneous, if
A laboratory reagent imparts green colour to the flame. On heating with solid K2Cr2O7 and conc. H2SO4, it evolves an orange red gas. Identify the reagent.
van’t Hoff factors are x, y and z in case of association, ionisation and no change, respectively. Their increasing order is
Which of the following does not illustrate the anomalous properties of Li ?
If for a reaction A→B,ΔH = —10 kJ mol—1 and Ea = 50 kJ mol—1, the energy of activation for reaction B→A will be
Reduction of a metal oxide by excess carbon at high temperature is a method for the commercial preparation of some metals. This method can be successfully applied in the case of
Elevation in boiling point of an aqueous urea solution is 0.520(kb = 0.520 mol—1 kg). Hence, mole fraction of urea in this solution is
A 100 mL sample is removed from water solution saturated with CaSO4 at 250C. The water is completely evaporated from the sample and a deposit of 0.24 g CaSO4 is obtained. The Ksp of CaSO4 at 250C is
In a transition series, as the atomic number increases, paramagnetism
When FeS2 is burnt in air, it converts to Fe2O3. The change in percentage by weight of iron in the process is (Fe = 56)


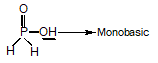
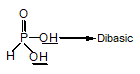
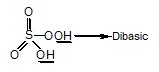
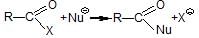 is fastest when X is
is fastest when X is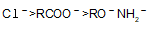
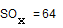
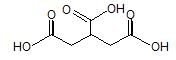

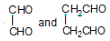 Olefin is
Olefin is