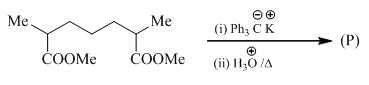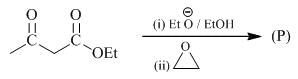IIT JAM Chemistry - MCQ Test 4 - Chemistry MCQ
30 Questions MCQ Test - IIT JAM Chemistry - MCQ Test 4
Relative lowering of vapor pressure produced by dissolving 71.5g of a substance in 1Kg of water is 0.0073. Molecular weight of substance will be
Which solution will show maximum vapor pressure at 300K
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The relationship been Osmotic pressure at 273K when 10g Glucose (P1), 10g Urea (P2), 10g Sucrose (P3) are dissolved in 250 Ml of water
Solution A contains 7g/L of MgCl2 and Solution B contains 7g/L of NaCl. At room temp, Osmotic pressure of
When a substance is dissolved in solvent, its vapor pressure is decreased. It brings
At a given temperature, total vapor pressure in Torr of a mixture of volatile components A and B is given by
p = 120 – 75XB
Hence, vapor pressure of pure A and B respectively (in Torr) are:
The equilibrium constant is 6.0 × 10–4 for the N2O2→ 2NO reaction. If the concentration of nitrogen is 0.10 mol/L and concentration of oxygen is 0.20 mol/L at equilibrium. Then the concentration of nitric oxide at equilibrium is:
Some solid NH4HS is placed in a flask containing 0.5 atm of NH3, what would be pressure of NH3 and H2S when equilibrium is reached:
NH4HS(g)→ NH3(g) + H2S(g), KP = 0.11
For the following three reactions (I), (II) and (III) equilibrium constants are given
(I) CO(g) + H2O(g)→ CO2(g) + H2(g); K1
(II) CH4(g) + H2O(g)→ CO(g) + 3H2(g); K2
(III) CH4(g) + 2H2O(g)→ CO2(g) + 4H2(g); K3
Which of the following relations is correct:
XY2 dissociates as XY2→ XY(g) + Y(g) when the initial pressure of XY2 is 600 mmHg, the total equilibrium pressure is 800mm Hg. Calculate K for the reaction assuming that the volume of the system remain unchanged:
A 50 mL sample of 0.01M Ba(OH)2 is titrated with 0.01 M HCl. The solution at equivalent point is
Based on the following information,
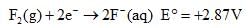
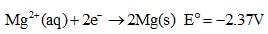
Which of the following chemical species is the strongest reducing agent?
Which of the following processes involves increasing in bond order:
At room temperature, HCl is a gas while HF is a liquid because:
The bond order and bond type in the C2 molecule are, respectively:
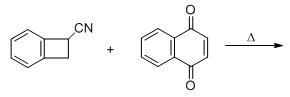
The major product formed in the following reacting is
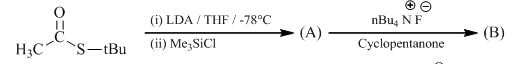
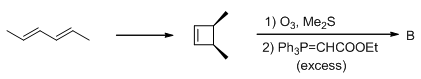
Predict the condition A and the structure of the major product B in the following sequence.
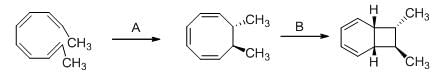
The conditions A-B, required for the following pericyclic are
Solubility of BaSO4 in aqueous solution is 1 × 10–5 M. Hence, solubility in 0.1 M BaCl2 is:
pH of a mixture of 1 M benzoic acid (pKa = 4.20) and 1 M sodium benzoate is 4.5. In 300 mL buffer, benzoic acid is:
58.5 g of NaCl and 180g of glucose were separately dissolved in 1L of water. Identify the correct statements regarding the elevation in boiling point of solution.
Elevation in boiling point of solution of 13.44g og CuCl2 in 1 Kg of water will be (M.wt = 134.4, Kb =0.52Kmolal-1, assume it is a strong electrolyte)
At 700 K, the equilibrium constant Kp, for the reaction 2 SO3 (g) ⇌ 2 SO2 (g) + O2 Is 1.8 X 10-3 kPa. What is the numerical value in moles per liter of Kc for the reaction at the same temperature?
0.1 mol of N2O4(g) was sealed in a tube under 1 atmospheric pressure at 25oC. Calculate the no of mole of NO2(g) present if equilibrium is reached after sometime (Kp = 0.14)