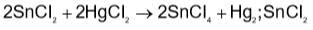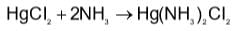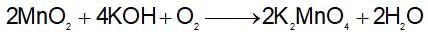Extraction & D & F Block - Class 12 MCQ
30 Questions MCQ Test - Extraction & D & F Block
Which of the following statements regarding the metallurgy of magnesium using electrolytic method is not correct?
The coordination number of copper in the complex formed by adding excess of NH3 to CuSO4 solution is:
Powdered silver ore is treated with NaCN solution and air is bubbled through the mixture to give:
Which method of purification is represented by the following equation?
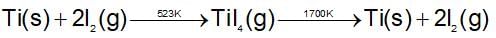
In the cyanide extraction process of silver from argentite ore, the oxidizing and reducing agents used are:
What effect is noticed on shaking dilute sulphuric acid with a small quantity of anhydrous copper sulphate?
In the purification of copper by electrolysis, which is incorrect?
In order to refine “blister copper” it is melted in a furnace and is stirred with green logs of wood. The purpose is:
When excess of sodium thiosulphate is added to dil. AgNO3 solution, a soluble compound X is formed. However, when dil. Na2S2O3 solution is added to conc. AgNO3 solution a white ppt., turning yellow and finally black ppt. of Y is obtained. Which is correct pair?
Which one of the following pairs of substances or reaction will not evolve H2 gas?
Before introducing FeO in blast furnace, it is converted to Fe2O3 by roasting so that:
Which of the following statements about the advantage of roasting of sulphide ore before reduction is not true?
In the commerical electrochemical process for aluminium extraction, electrolyte used is:
Match the extraction process listed in column I with metals listed in column II.
Column I Coumn II
A. Self-reduction (P) Lead
B. Carbon reduction (Q) Silver
C. Complex formation and (R) Copper
displacement by metal
D. Decomposition of iodide (S) Boron
In context with the transition elements, which of the following statements is incorrect?
A yellow ppt. is formed when H2S is passed through an acidified solution of :
The inner transition elements are the elements in which the added electrons go to:



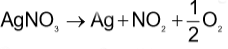
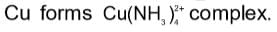
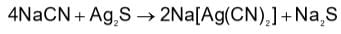
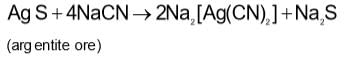

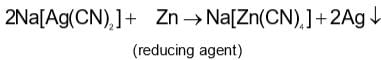
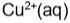 blue in colour
blue in colour 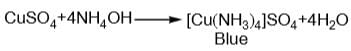
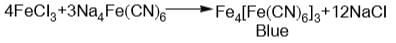

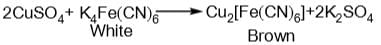
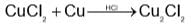
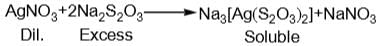
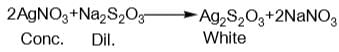
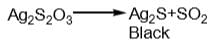
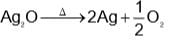
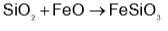
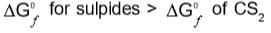 and H2S and thus, C and H2 cannot reduce metal sulphide.
and H2S and thus, C and H2 cannot reduce metal sulphide.