Basic Mathematical And Analytical Chemistry - Chemistry MCQ
30 Questions MCQ Test - Basic Mathematical And Analytical Chemistry
The alkaline earth metal that imparts green color to Bunsen flame when introduced in it in the form of its chloride is:
The metal does not give borax bead test is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the measurement of hardness of water by complexometric titration, identify P and Q in the following equation.[In = Indicator]:
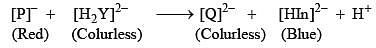
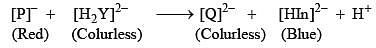
A gas ‘X’ is passed through water to form a saturated solution. The aqueous solution on treatment with silver nitrate gives a white precipitate. The saturated aqueous solution also dissolves magnesium ribbon with evolution of a colorless gas ‘y’. Identify ‘X’ and ‘Y’:
Brown ring test for nitrates depends upon:
Find the eigen value of the following 3 × 3 matrix given that 2 is one of the eigen values. Compute the determinant of the matrix using the eigen values:

For square matrices M and N, if NM = M and NM = N, then:
Which one of the following is an identity matrix?
A reagent used to test presence of Fe+2 ion is:
An aqueous solution of substance gives a white precipitate on treatment with dil. HCl which dissolves on heating. When H2S is passed through the hot acidic solution a black ppt. is obtained.The substance is:
Bi(dmg)2 complex has following color?
When H2S gas is passed through the HCl containing aqueous solution of CuCl2, HgCl2, BiCl3 and CoCl2, which does not precipitate out:
A mixture when heated with conc H2SO4 with MnO2 brown fumes are formed due to:
X and Y transformed co-ordinates obtained from p and q as follows:

The correct set of linear equations that represent X and Y are:
Two matrices are given as X 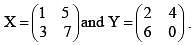 If XT is the transpose of X, then, calculate XTY?
If XT is the transpose of X, then, calculate XTY?
The scalar product of two vectors u and v, where u 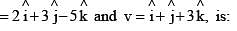
The function y = x exp (-x2 ) has a minimum at x =- 1/√2. The second derivation of the function at the minimum is:
What product is formed by mixing the solution of K4[Fe(CN)6] with the solution of FeCl3 :
Which is the correct structure of Alizarin adsorption indicator:
Mn+2 ions in presence of S2– gives a precipitate of following color?
A vector 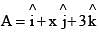 is rotated through an angle and is also doubled in magnitude resulting in
is rotated through an angle and is also doubled in magnitude resulting in 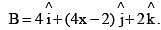 An acceptable value of x is:
An acceptable value of x is:

 where h, π, m and α are constant. C is:
where h, π, m and α are constant. C is:
The maximum of a function Ae-ax2 (A < 0, a> 0) is at x = ?
Which of the following is not a solution of the equation:
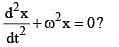




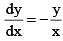 is a differential equation for a/an:
is a differential equation for a/an:















