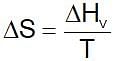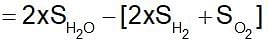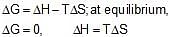Thermodynamics - Class 12 MCQ
30 Questions MCQ Test - Thermodynamics
The formation of water from H2(g) and O2(g) is an exothermic reaction because:
The enthalpy changes of formation of the gaseous oxides of nitrogen (N2O nd NO) are positive because of:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The temperature of a 5 mL of strong acid increases by 50C when 5 mL of a strong base is added to it, If 10 mL of each are mixed, temperature should increase by:
The value of ΔH0 for the reaction Cu+(g) + l-(g) → Cul(g) is -446 kJ mol-1. If the ionisation energy of Cu(g) is 745 kJ mol-1 and the electron affinity of I(g) is -295 kJ mol-1, then the value of ΔH0 for the formation of one mole of CuI(g) from Cu(g) and l(g) is:
The heat of combustion for C,H2 and CH4 are -349.0,-241.8 and -906.7 kJ respectively. The heat of formation of CH4 is:
Equal volume of M HCI and 1 M H2SO4 are neutralised by dilute NaOH solution and x and y kcal of heat are liberated respectively. Which of the following is true?
When a certain amount of ethyene was burnt, 6226 kJ was evolved. If heat of combustion of ethylene is 1411 kJ, the volume of O2 (at NTP) that entered into the reaction is:
Heat of combusion ΔH for C(s),H2(g) and CH4(g) are -94, -68 and -213 kcal/mole,than
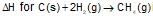 is:
is:
Assume each reaction is carried out in an open container. For which reaction will ΔH = ΔU?
ΔHf of graphite is 0.23 kJ/mol and ΔHf of diamond is 1.896 kJ/mol. ΔHTrsnsition from graphite to diamond is:
For the reaction
X2O4(l) → 2XO2(g)
ΔU = 2.1 kcal, ΔS = 20 cal K-1 at 300K
Hence,ΔG is:
A container has hydrogen and oxygen mixture in ratio of 4:1 by mass, then on mixing:
Which of the following is correct option for free expansion of an ideal gas ?
Enthalpy of vaporisation for water is 186.5 kJ mol-1. The entropy change during vaporisation is ...kJ K-1mol-1
The work done by a system is 8 J, when 40 J heat is supplied to it. The change in internal energy of the system during the process is:
The maximum work done in expanding 16 g oxygen at 300 K and occupying a volume of 5 dm3 isothermally until the volume become 25dm3 is:
The entropy change for the reaction given below,
2H2(g) + O2 (g) → 2H2O(l)
is....at 300 K. Standard entropies of H2(g),O2(g) and H2O(l) are 126.6, 201.20 and 68.0 JK-1 mol-1 respectively.
One mole of ice is converted into water at 273 K. The entropies of H2O(s) and H2O(l) are 38.20 and 60.01 J mol-1 K-1 respectively. The enthalpy change for the conversion is:
Boiling point of a liquid is 50 K at 1 atm and ΔH vap= 460.6 cal mol-1 . What will be its b.p. at 10 atm?
The ratio of slopes of log P vs log V for reversible adiabatic process and reversible isothermal process of an ideal gas is equal to :
Internal energy and pressure of a gas of unit volume are related as:
The molar heat capacity of water at constant pressure P, is 75 JK-1mol-1. When 1.0 kJ of heat is supplied to 100 g of water which is free to expand,the increase in temperature of water is :


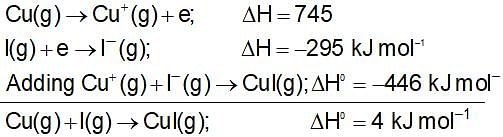
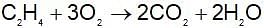
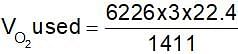
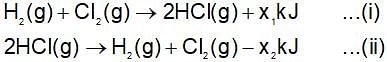
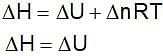
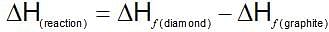
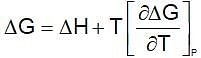 (Gibbs - Helmholtz equation)
(Gibbs - Helmholtz equation)
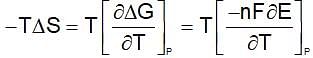
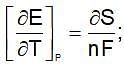 Similarly drive for other values
Similarly drive for other values