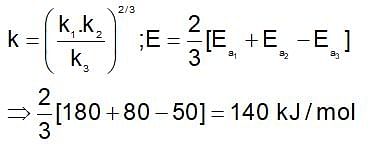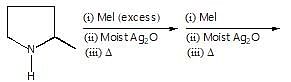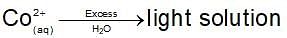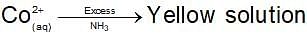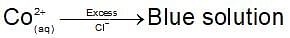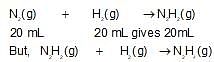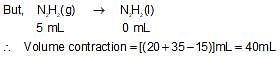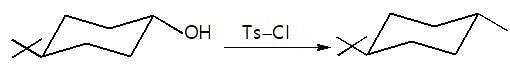Mock Test -3 - Class 12 MCQ
30 Questions MCQ Test - Mock Test -3
If the H+ ion concentration is decreased 100 times in MnO-4 / Mn+2couple then oxidising power of this couple. {Taking concentration of all other ion unity, RT/F = 0.06]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
First ionisation energy of an electron in the ground state of He-atom is equal to 24 eV/atom. Then calculate the amount of energy required to convert a He-atom in a-particle?
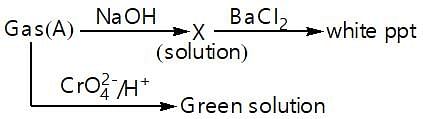
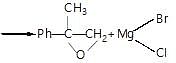
Which of the following statement stands True for the following reaction
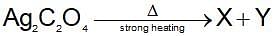
The equilibrium constant for the reaction: 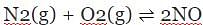 at 200 K is 4 x10-4. In presence of catalyst, equilibrium is attained 5 times faster, then value of equilibrium constant at 200 K in presence of catayst will be
at 200 K is 4 x10-4. In presence of catalyst, equilibrium is attained 5 times faster, then value of equilibrium constant at 200 K in presence of catayst will be
If the closest distance between cation and anion in the unit cell of sodium- chloride is 300 pm then final the distance of body-diagonal in unit cell of sodium-chloride.
Which of the following set, povides the crystal field stablization energy equals to-1.2 Δ0 for octahedral complex?
Find the pH of solution which is formed by mixing 50 mL of 0.1 M H2SO4(aq) solution with 100 mL of 0.15 M NH4OH(aq) solution?[Given : Kb(NH4OH) = 10-5]
Which of the following configuration represent increase in anion formation ability
One mole of Ar(g) is compressed isothermally and reversibly at 270 C to half of its original volume, then select the incorrect statement(s)-[In 2=0.7,R=2 cal/mol-K]
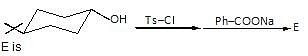
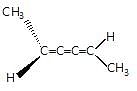
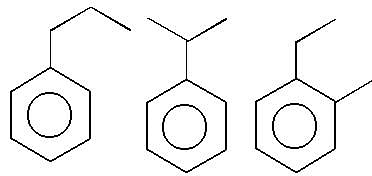
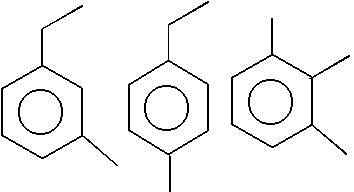
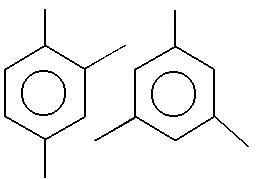
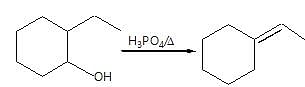
Observe the pair of structure & select the option in which both the structures does not represents the same compound
The freezing point of an aqueous solution containing 0.1 g of K3[Fe(CN)6] (M.W. = 329 g/mol) in 100 g water (Kf = 1.86 K-Kg mol-1] will be-
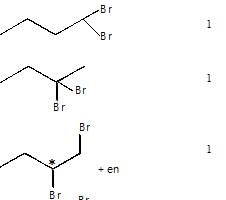
How many fraction can be obtained by fractional distillation of mixture formed by dibromination of n-butane?
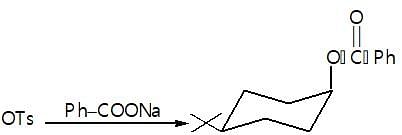
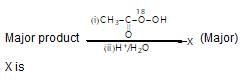
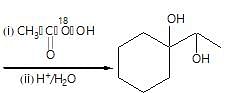
Observe the following reaction
 Dark blue solution
Dark blue solution
Find the ligand for the product
N2(g) and H2(g) combines to form N2H2(l) and it is also known that N2H2(l) can be converted to N2H4(g) by mixing it with H2(g). If 20 mL N2(g) and 35 mL H2(g) are mixed togther then volume contraction after completion of reaction is:
Orange soild(s)  paramagnetic gas+green solid+yellow solid
paramagnetic gas+green solid+yellow solid
Identify the orange soild & green solid in the above reaction?


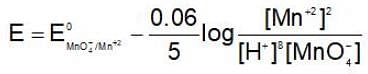
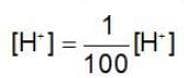
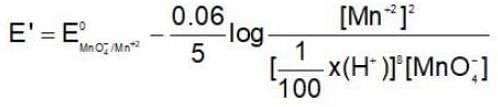
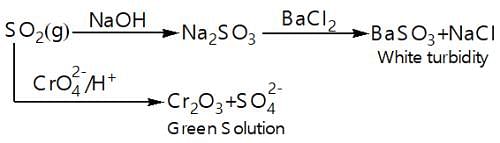


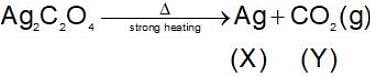


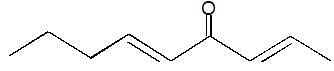
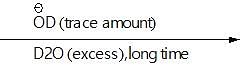

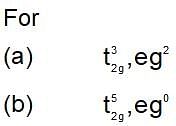

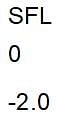
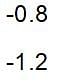
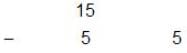
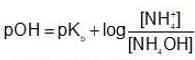
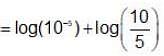
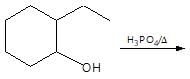


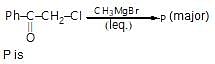


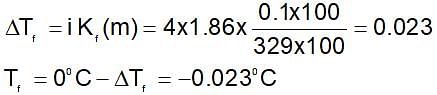

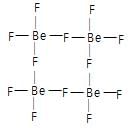


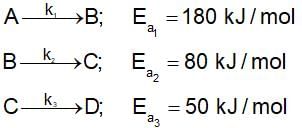
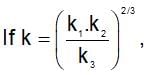 then the activation energy
then the activation energy