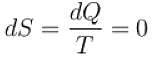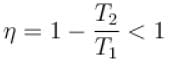Second Law Of Termodynamics – MSQ - Physics MCQ
10 Questions MCQ Test - Second Law Of Termodynamics – MSQ
Which of the following is/are correct statement?
Select one or more:
Select one or more:
Entropy does not remains constant in which of the following processes?
Select one or more:
Select one or more:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The main cause of irreversibility is/are.
Select one or more:
Select one or more:
Which of the following is a property of entropy?
Select one or more:
A Carnot cycle operates on a working substance between two reservoirs at temperature T1 and T2, where T1 >T2. During each cycle, an amount of heat Q1 is extracted from the reservoir at T1 and an amount Q2 is delivered to the reservoir at T2. Which of the following statements are correct?
Select one or more:
The condition for the reversibility of a cycle is.
Select one or more:
Which of the following statements are correct?
Select one or more:
For any process, the second law of thermodynamics requires that the change of entropy of the universe be.
Select one or more:
Out of three Carnot engines, operating between reservoir temperature of (a) 400K and 500K (b) 600K and 800K (c) 800K and 1000K, which has/have the lowest thermal efficiency?
Select one or more:
According to Carnot’s theorem.
Select one or more: